
Identify the reaction and complete


Answer
573.3k+ views
Hint: The given reaction is a hydration reaction where water is added to the alkyne. Alkyne reacting in presence of dilute sulphuric acid and mercuric sulphate gives aldehyde and ketone as the main product.
Complete answer:
Hydration reaction is a type of reaction when the reactant is treated with water.
In this reaction, hydration of alkyne takes place.
The reaction of but-1-yne with water is shown below.
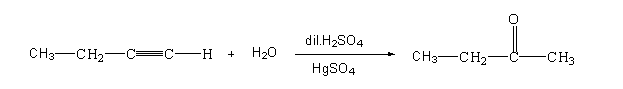
In this reaction, but-1-yne reacts with water in presence of dilute sulphuric acid and mercuric sulphate to form butan-2-one.
In this reaction, the water molecules attack the triple bond. The intermediate product which is formed is highly unstable. After that the triple bond is changed to double bond. As soon the intermediate compound rearrange itself and gets converted to the carbonyl compound, the hydration of But-1-yne will form butan-2-one which is a ketone compound.
The hydration of alkyne is difficult as compared to the hydration of alkene. In this reaction the dilute sulphuric acid and mercuric sulphate is used as a catalyst which does not take part in the reaction but only helps to increase the reaction rate.
In the alkene hydration reaction, only dilute sulphuric acid is utilized but in the hydration of alkyne mercuric sulphate is used with dilute sulphuric acid because the reaction rate of alkyne and sulphuric acid is very slow.
Note:
In the hydration of alkyne, only ethyne is the compound which gives aldehyde as a main product otherwise all the other alkyne with higher hydrocarbons gives ketone as the product.
Complete answer:
Hydration reaction is a type of reaction when the reactant is treated with water.
In this reaction, hydration of alkyne takes place.
The reaction of but-1-yne with water is shown below.
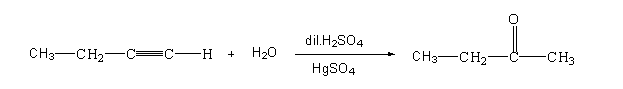
In this reaction, but-1-yne reacts with water in presence of dilute sulphuric acid and mercuric sulphate to form butan-2-one.
In this reaction, the water molecules attack the triple bond. The intermediate product which is formed is highly unstable. After that the triple bond is changed to double bond. As soon the intermediate compound rearrange itself and gets converted to the carbonyl compound, the hydration of But-1-yne will form butan-2-one which is a ketone compound.
The hydration of alkyne is difficult as compared to the hydration of alkene. In this reaction the dilute sulphuric acid and mercuric sulphate is used as a catalyst which does not take part in the reaction but only helps to increase the reaction rate.
In the alkene hydration reaction, only dilute sulphuric acid is utilized but in the hydration of alkyne mercuric sulphate is used with dilute sulphuric acid because the reaction rate of alkyne and sulphuric acid is very slow.
Note:
In the hydration of alkyne, only ethyne is the compound which gives aldehyde as a main product otherwise all the other alkyne with higher hydrocarbons gives ketone as the product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




