
Identify the pair in which the first structure is more stable than the second structure.
(A) 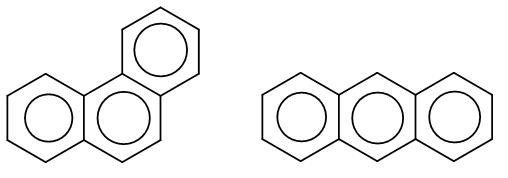
(B) 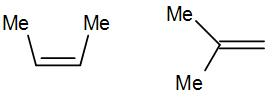
(C) 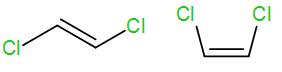
(D) 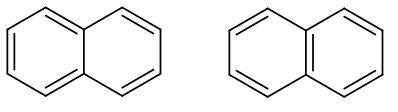
Answer
233.1k+ views
Hint: There are different factors that affect the stability of an organic compound. Here you have to consider the factors of resonance stabilization due to the formation of benzene like structures. Also, you have to consider the factors affecting the stability of an alkene.
Complete step by step solution:
To find the correct answer to this question, let us discuss the pairs in each of the options one by one.
Firstly, we have-
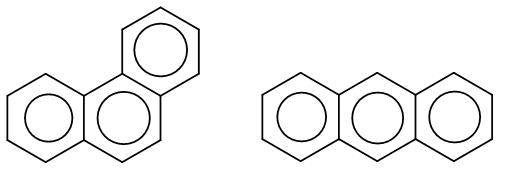
Here, the first structure is phenanthrene and the second structure is anthracene. Among these two there is an aromaticity difference which makes one of the more stable than the other.
In phenanthrene, the pi bonding is more efficient and it has two sextets whereas anthracene has just one sextet which makes phenanthrene more stable.
Then we have
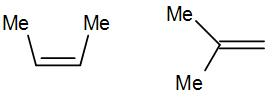
Here we have cis-alkene in the first structure and a geminal alkene i.e. the methyl groups are substituted to the same carbon.
The geminal structure is more stable than the cis or even trans alkene. The more substituted alkene is more stable according to Saytzeff’s rule a thus geminal structure is more stable than the cis structure.
Then we have
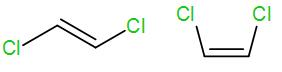
We know that trans alkene is more stable than cis alkenes. Here the first structure is a trans alkene and the second structure is a cis alkene.
The higher stability of trans alkene is due to lesser steric interaction among the substituted groups. In the cis structure, there is greater steric interaction between the two groups and thus causes de-stability.
And lastly, we have-
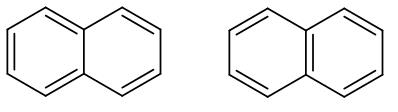
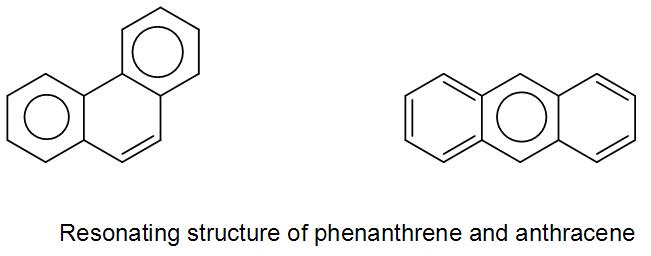
These are basically the two resonance structures of naphthalene. As we can see in both the structures the double bond is in conjugation. However, in the second structure as we can see there are two benzene rings therefore it has higher resonance energy compared to the first structure.
We can see from the above discussion that the correct answers are options (A) and (C).
Note: Saytzeff’s rule, also known as Zaitsev’s rule is a rule in organic chemistry which is used to find out the favoured alkene product in an elimination reaction. In a variety of elimination reactions, a general trend was observed in the resulting alkenes. Based on this general trend of the alkene products, Saytzeff’s rule was coined. We can write the actual statement of the rule as- "The alkene formed in the greatest amount is the one that corresponds to the removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents."
Complete step by step solution:
To find the correct answer to this question, let us discuss the pairs in each of the options one by one.
Firstly, we have-

Here, the first structure is phenanthrene and the second structure is anthracene. Among these two there is an aromaticity difference which makes one of the more stable than the other.
In phenanthrene, the pi bonding is more efficient and it has two sextets whereas anthracene has just one sextet which makes phenanthrene more stable.

Then we have
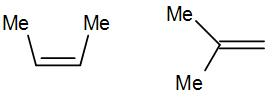
Here we have cis-alkene in the first structure and a geminal alkene i.e. the methyl groups are substituted to the same carbon.
The geminal structure is more stable than the cis or even trans alkene. The more substituted alkene is more stable according to Saytzeff’s rule a thus geminal structure is more stable than the cis structure.
Then we have

We know that trans alkene is more stable than cis alkenes. Here the first structure is a trans alkene and the second structure is a cis alkene.
The higher stability of trans alkene is due to lesser steric interaction among the substituted groups. In the cis structure, there is greater steric interaction between the two groups and thus causes de-stability.
And lastly, we have-

These are basically the two resonance structures of naphthalene. As we can see in both the structures the double bond is in conjugation. However, in the second structure as we can see there are two benzene rings therefore it has higher resonance energy compared to the first structure.
We can see from the above discussion that the correct answers are options (A) and (C).
Note: Saytzeff’s rule, also known as Zaitsev’s rule is a rule in organic chemistry which is used to find out the favoured alkene product in an elimination reaction. In a variety of elimination reactions, a general trend was observed in the resulting alkenes. Based on this general trend of the alkene products, Saytzeff’s rule was coined. We can write the actual statement of the rule as- "The alkene formed in the greatest amount is the one that corresponds to the removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents."
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




