
i) Write the equations for the steps in \[{{S}_{N}}_{1~}\] mechanism of the conversion of tert.butyl bromide into tert.butyl alcohol.
ii) Haloarenes are less reactive towards nucleophilic substitution reactions than Haloalkanes. Give a reason.
b) Complete the following equations:
i) \[{{C}_{2}}{{H}_{5}}OH+SOC{{l}_{2}}\to \] ...........................
ii) Please find the above image.
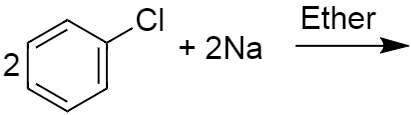
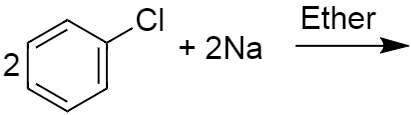
Answer
573.6k+ views
Hint:There are two parts of the question each having two subparts. In the nucleophilic substitution reaction formation of a carbocation intermediate takes place.
-The reactivity of haloalkanes and haloarenes depends on how easy it is to break the bond between carbon and the halogen group attached to it.
Complete answer:
a) i)The \[{{S}_{N}}_{1~}\] reaction can be defined as a type of nucleophilic substitution reaction where the rate determining step is unimolecular. It is a kind of organic substitution reaction. \[{{S}_{N}}_{1~}\] stands for substitution nucleophilic unimolecular. Thus, the rate equation which says that the \[{{S}_{N}}_{1~}\] reaction is dependent on the electrophile present in the reactant and not on the nucleophile, is applicable in situations where the quantity of the nucleophile is far greater than the amount of the carbocation intermediate which is being formed during the reaction.
-In this reaction formation of a carbocation intermediate takes place. It is generally being observed in case of the reactions of secondary or tertiary alkyl halides with tertiary or secondary alcohols in conditions which are strongly acidic or strongly basic in nature. The \[{{S}_{N}}_{1~}\] reaction is frequently referred to as the dissociative mechanism in the field of inorganic chemistry.
-The equations for the steps in \[{{S}_{N}}_{1~}\] mechanism of the conversion of tertbutylbromide into tertbutylalcohol are given below. \[{{\left( C{{H}_{3}} \right)}_{3}}C-Br\to {{\left( C{{H}_{3}} \right)}_{3}}{{C}^{+}}+B{{r}^{-}}\] \[{{\left( C{{H}_{3}} \right)}_{3}}{{C}^{+}}+O{{H}^{-}}\to {{\left( C{{H}_{3}} \right)}_{3}}CBr\]
ii) Haloalkanes are more reactive towards nucleophilic substitution reactions than Haloarenes. This is because In haloarenes, the bond between the carbon and the functional group that is \[C-X~\] has a partial double bond character as the lone pair of electrons on halogen is involved in the process of resonance with the benzene ring. Hence, \[C-X~\] bond is difficult to break in haloarenes. While in the case of haloalkanes, the \[C-X~\] bond is just a single bond which is much easier to break.
b)i) The complete reaction is written below,
\[{{C}_{2}}{{H}_{5}}OH+SOC{{l}_{2}}\to {{C}_{2}}{{H}_{5}}-Cl+S{{O}_{2}}\downarrow +HCl\downarrow \]
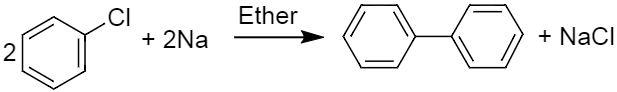
ii) Given below is the complete reaction, which was being asked in the question.
It is an example of fittig reaction in which two molecules of aryl halide react with sodium metal in presence of dry ether to give substituted aromatic compounds .
Note:
-The haloarenes show resonance effect because of the presence of alternate double bonds in the ring.
-The resonating effect makes it more stable, because the electron clouds are more delocalised, hence they are less reactive than the haloalkanes where the electron cloud is more localised and the bonds are easy to break.
-The reactivity of haloalkanes and haloarenes depends on how easy it is to break the bond between carbon and the halogen group attached to it.
Complete answer:
a) i)The \[{{S}_{N}}_{1~}\] reaction can be defined as a type of nucleophilic substitution reaction where the rate determining step is unimolecular. It is a kind of organic substitution reaction. \[{{S}_{N}}_{1~}\] stands for substitution nucleophilic unimolecular. Thus, the rate equation which says that the \[{{S}_{N}}_{1~}\] reaction is dependent on the electrophile present in the reactant and not on the nucleophile, is applicable in situations where the quantity of the nucleophile is far greater than the amount of the carbocation intermediate which is being formed during the reaction.
-In this reaction formation of a carbocation intermediate takes place. It is generally being observed in case of the reactions of secondary or tertiary alkyl halides with tertiary or secondary alcohols in conditions which are strongly acidic or strongly basic in nature. The \[{{S}_{N}}_{1~}\] reaction is frequently referred to as the dissociative mechanism in the field of inorganic chemistry.
-The equations for the steps in \[{{S}_{N}}_{1~}\] mechanism of the conversion of tertbutylbromide into tertbutylalcohol are given below. \[{{\left( C{{H}_{3}} \right)}_{3}}C-Br\to {{\left( C{{H}_{3}} \right)}_{3}}{{C}^{+}}+B{{r}^{-}}\] \[{{\left( C{{H}_{3}} \right)}_{3}}{{C}^{+}}+O{{H}^{-}}\to {{\left( C{{H}_{3}} \right)}_{3}}CBr\]
ii) Haloalkanes are more reactive towards nucleophilic substitution reactions than Haloarenes. This is because In haloarenes, the bond between the carbon and the functional group that is \[C-X~\] has a partial double bond character as the lone pair of electrons on halogen is involved in the process of resonance with the benzene ring. Hence, \[C-X~\] bond is difficult to break in haloarenes. While in the case of haloalkanes, the \[C-X~\] bond is just a single bond which is much easier to break.
b)i) The complete reaction is written below,
\[{{C}_{2}}{{H}_{5}}OH+SOC{{l}_{2}}\to {{C}_{2}}{{H}_{5}}-Cl+S{{O}_{2}}\downarrow +HCl\downarrow \]
ii) Given below is the complete reaction, which was being asked in the question.
It is an example of fittig reaction in which two molecules of aryl halide react with sodium metal in presence of dry ether to give substituted aromatic compounds .

Note:
-The haloarenes show resonance effect because of the presence of alternate double bonds in the ring.
-The resonating effect makes it more stable, because the electron clouds are more delocalised, hence they are less reactive than the haloalkanes where the electron cloud is more localised and the bonds are easy to break.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




