
(i) Why is a cathode ray tube evacuated to a low pressure?
(ii) What happens if the negative potential is changed on a grid?
Answer
478.5k+ views
Hint: We know that in normal pressure conditions, air molecules are present in abundance. These molecules keep randomly moving around and cause deflection in moving particles. Also, in a grid, the negative potential supplied plays a major role.
Complete answer:
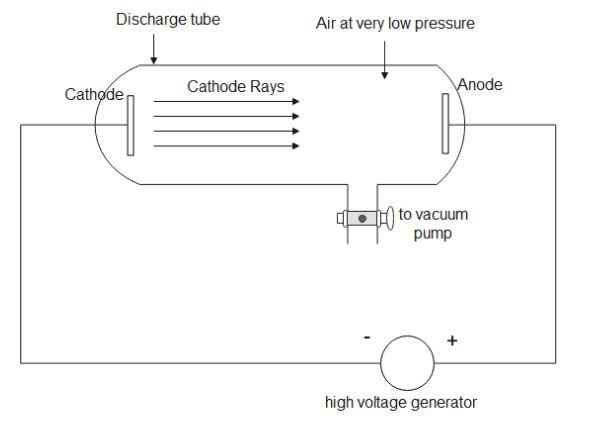
A Cathode ray tube setup consists of a discharge tube which is connected to the vacuum pump. There is an anode and a cathode inside the tube, which is connected to a high voltage generator. When this setup is switched on, a faint green luminance is produced behind the anode where the cathode rays strike.
(i) The cathode ray tube is evacuated to a low pressure so that the electrons can move freely through the tube. In the presence of normal pressure, the electrons are obstructed by the air molecules.
(ii) If the negative potential is changed on the grid, the number of electrons reaching the anode will fluctuate. This in turn will cause the number of electrons reaching the anode to change. Thus, the green luminance behind the anode will change its pattern accordingly.
Note: For better results in cathode ray experiment, an evacuated (low pressure) discharge tube is filled up with hydrogen gas. Hydrogen gas is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio.
Complete answer:

A Cathode ray tube setup consists of a discharge tube which is connected to the vacuum pump. There is an anode and a cathode inside the tube, which is connected to a high voltage generator. When this setup is switched on, a faint green luminance is produced behind the anode where the cathode rays strike.
(i) The cathode ray tube is evacuated to a low pressure so that the electrons can move freely through the tube. In the presence of normal pressure, the electrons are obstructed by the air molecules.
(ii) If the negative potential is changed on the grid, the number of electrons reaching the anode will fluctuate. This in turn will cause the number of electrons reaching the anode to change. Thus, the green luminance behind the anode will change its pattern accordingly.
Note: For better results in cathode ray experiment, an evacuated (low pressure) discharge tube is filled up with hydrogen gas. Hydrogen gas is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




