
What is the hybridization of Carbon atoms present in haloarenes?
(A) $SP$
(B) $S{{P}^{2}}$
(C) $S{{P}^{3}}$
(D) $S{{P}^{3}}d$
Answer
573.3k+ views
Hint: This question is based on VSEPR theory. In haloarenes each carbon atom forms one $\pi $ bond and as carbon is tetravalent so remaining three bonds will be sigma bonds and also carbon doesn’t have a lone pair, so its degree of hybridization will be three and the ring will be having planar structure.
Complete answer:
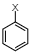
This is the structure of haloarene where one can clearly observe each carbon atom and find that it is forming one pi bond and three sigma bonds. Electronic configuration of carbon atom
\[1{{S}^{2}}2{{S}^{2}}2{{P}^{2}}\]
One electron of 2S orbital shifts into vacant 2P orbital so carbon can be tetravalent. So new electronic configuration of carbon atom
$1{{S}^{2}}2{{S}^{1}}2{{P}^{3}}$
Now carbon has four half filled orbitals, out of which one P-orbital takes part in pi-bond formation and remaining three will form sigma bonds. Among the orbitals those are forming sigma bonds, one is S orbital and other two are P orbitals.
Number of sigma bonds= 3
Number of lone pairs=0
Degree of hybridization= Number of sigma bonds + Number of lone pairs
Degree of hybridization= 3+0
Degree of hybridization=3
As the degree of hybridization is three and one S and 2 P orbitals are taking part in sigma bond formation, that’s why hybridization of carbon atoms over here is SP.
Correct answer is Option (A)
Additional information: Always remember that the formula we used is only applicable for covalent compounds, not for coordination compounds because in coordination compounds we use crystal field theory to explain the structure of them and VSEPR theory is not applicable over there.
Note: VSEPR theory accounts for the presence of lone pairs and its impact on the structure of molecules. It considers that the presence of lone pair distorts the structure because lone pair repels bond pairs more than a bond pair repels a bond pair and that readjustment within the molecule takes place to minimize the repulsion and that alters the bond angle.
Complete answer:

This is the structure of haloarene where one can clearly observe each carbon atom and find that it is forming one pi bond and three sigma bonds. Electronic configuration of carbon atom
\[1{{S}^{2}}2{{S}^{2}}2{{P}^{2}}\]
One electron of 2S orbital shifts into vacant 2P orbital so carbon can be tetravalent. So new electronic configuration of carbon atom
$1{{S}^{2}}2{{S}^{1}}2{{P}^{3}}$
Now carbon has four half filled orbitals, out of which one P-orbital takes part in pi-bond formation and remaining three will form sigma bonds. Among the orbitals those are forming sigma bonds, one is S orbital and other two are P orbitals.
Number of sigma bonds= 3
Number of lone pairs=0
Degree of hybridization= Number of sigma bonds + Number of lone pairs
Degree of hybridization= 3+0
Degree of hybridization=3
As the degree of hybridization is three and one S and 2 P orbitals are taking part in sigma bond formation, that’s why hybridization of carbon atoms over here is SP.
Correct answer is Option (A)
Additional information: Always remember that the formula we used is only applicable for covalent compounds, not for coordination compounds because in coordination compounds we use crystal field theory to explain the structure of them and VSEPR theory is not applicable over there.
Note: VSEPR theory accounts for the presence of lone pairs and its impact on the structure of molecules. It considers that the presence of lone pair distorts the structure because lone pair repels bond pairs more than a bond pair repels a bond pair and that readjustment within the molecule takes place to minimize the repulsion and that alters the bond angle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




