
Glyptal polymer is obtained by the monomers:
(A)- malonic acid + ethylene glycol.
(B)- phthalic acid + ethylene glycol.
(C)- maleic acid + formaldehyde.
(D)- acetic acid + phenol.
Answer
593.7k+ views
Hint:
Polymers are formed by joining together through covalent bonds, a large number of simple repeating structural units derived from same or different small molecules called monomers. Repeating unit in glyptal is obtained by condensation of dibasic acids (or their anhydrides) with dihydric or trihydric alcohols.
Complete step by step answer:
Glyptal are polyesters formed by the condensation of organic acids having two acid functional groups such as phthalic acid, maleic acids, etc and alcohols with two or more hydroxyl groups. They are also called alkyd resins.
From the options given above, we can rule out the options (C) and (D). Since in option (C), there is no polyhydric alcohol and option (D) has acetic acid which is monobasic and phenol.
Malonic acid (\[COOH-C{{H}_{2}}-COOH\]) and ethylene glycol given in option (A) do not form polyester. Though malonic acid is a saturated dibasic acid, it is very unstable and heating gives acetic acid.
The simplest glyptal is formed by the polymerization of dibasic phthalic acid and dihydric alcohol, i.e., ethylene glycol with removal of water as a byproduct. The polymer thus formed is called poly (ethylene phthalate).
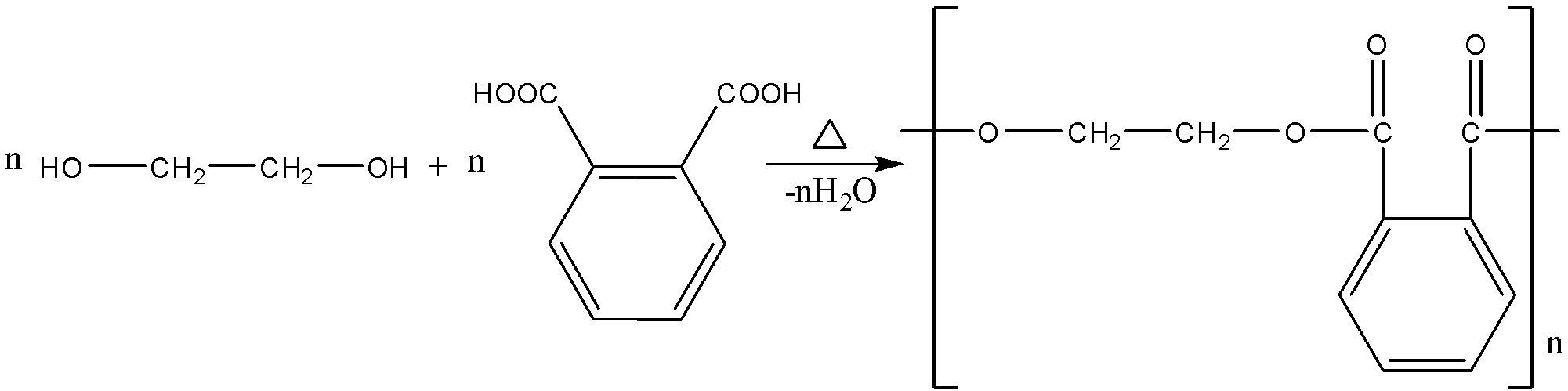
Therefore, the correct answer is option (B).
Additional Information:
Glyptal (polyethylene phthalate) is a thermoplastic. It, when dissolved in suitable solvents, forms a solution which evaporates from a tough, inflexible film. This property of glyptal is exploited to manufacture paints and lacquers.
Note:
Note that two monomers, i.e. phthalic acid and ethylene glycol undergo condensation reaction with the elimination of water, to form a unit which is repeated in regular fashion to obtain glyptal polymer. Sometimes, trihydric alcohol, i.e. glycerol is used instead of ethylene glycol along with phthalic acid in the condensation polymerization reaction to form glyptal.
Polymers are formed by joining together through covalent bonds, a large number of simple repeating structural units derived from same or different small molecules called monomers. Repeating unit in glyptal is obtained by condensation of dibasic acids (or their anhydrides) with dihydric or trihydric alcohols.
Complete step by step answer:
Glyptal are polyesters formed by the condensation of organic acids having two acid functional groups such as phthalic acid, maleic acids, etc and alcohols with two or more hydroxyl groups. They are also called alkyd resins.
From the options given above, we can rule out the options (C) and (D). Since in option (C), there is no polyhydric alcohol and option (D) has acetic acid which is monobasic and phenol.
Malonic acid (\[COOH-C{{H}_{2}}-COOH\]) and ethylene glycol given in option (A) do not form polyester. Though malonic acid is a saturated dibasic acid, it is very unstable and heating gives acetic acid.
The simplest glyptal is formed by the polymerization of dibasic phthalic acid and dihydric alcohol, i.e., ethylene glycol with removal of water as a byproduct. The polymer thus formed is called poly (ethylene phthalate).

Therefore, the correct answer is option (B).
Additional Information:
Glyptal (polyethylene phthalate) is a thermoplastic. It, when dissolved in suitable solvents, forms a solution which evaporates from a tough, inflexible film. This property of glyptal is exploited to manufacture paints and lacquers.
Note:
Note that two monomers, i.e. phthalic acid and ethylene glycol undergo condensation reaction with the elimination of water, to form a unit which is repeated in regular fashion to obtain glyptal polymer. Sometimes, trihydric alcohol, i.e. glycerol is used instead of ethylene glycol along with phthalic acid in the condensation polymerization reaction to form glyptal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




