
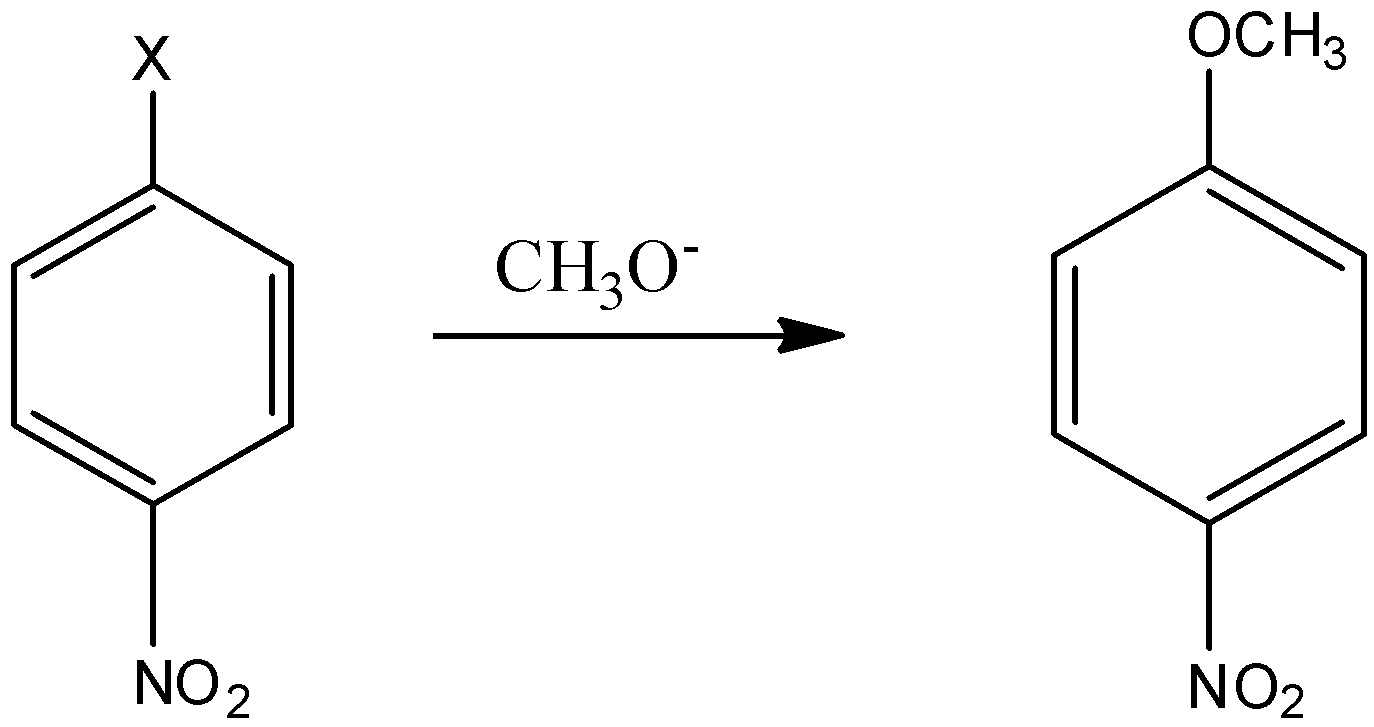
Given reaction is an example of nucleophilic aromatic substitution. Which of the following halide ${\text{( - X)}}$ is most readily replaced?
A) ${\text{ - F}}$
B) ${\text{ - Cl}}$
C) ${\text{ - Br}}$
D) ${\text{ - I}}$

Answer
573.3k+ views
Hint:In a nucleophilic aromatic substitution reaction, the nucleophile replaces the leaving group in the reagent structure. The leaving capacity of a leaving group or element depends on the stability of that group or element after leaving the reagent structure. One can think about the options and which can form a stable anion after it leaves.
Complete answer:
1) First of all, we will learn about the nucleophilic aromatic substitution reaction where the nucleophile attacks the carbon atom which has the electronegative atom attached to it which is leaving the group.
2) Now In the above reaction, the nucleophile is $C{H_3}{O^ - }$ which will attack the carbon-containing halogen group. The halide which readily gets replaced will be the halide which has better leaving ability.
3) The leaving atom or group ability is dependent on the stability of the anion formed by the leaving group. The stability of an anion depends on the electronegativity of that element.
4) The more the electronegativity of an element is the more will be its capacity to hold the anionic negative charge on itself. As more, the capacity of an element to hold the charge on the element the more will be its stability and it will act as a better leaving group.
5) Now the element which has the highest electronegativity among the given options is fluorine. Hence, the fluorine will be the good leaving group and form a stable anion after leaving. The correct order for the leaving group will be ${\text{Fe > Cl > Br > I}}$.
Therefore, the correct answer will be ${\text{ - F}}$ which shows option A as the correct choice.
Note:
The carbon atom which has halogen attached to it is electron-deficient carbon due to electronegative halogen and that is the main reason why nucleophilic attack takes place. The rate-determining step in this reaction is the attack of the nucleophile on the aromatic ring carbon-containing leaving group i.e. halogen element.
Complete answer:
1) First of all, we will learn about the nucleophilic aromatic substitution reaction where the nucleophile attacks the carbon atom which has the electronegative atom attached to it which is leaving the group.
2) Now In the above reaction, the nucleophile is $C{H_3}{O^ - }$ which will attack the carbon-containing halogen group. The halide which readily gets replaced will be the halide which has better leaving ability.
3) The leaving atom or group ability is dependent on the stability of the anion formed by the leaving group. The stability of an anion depends on the electronegativity of that element.
4) The more the electronegativity of an element is the more will be its capacity to hold the anionic negative charge on itself. As more, the capacity of an element to hold the charge on the element the more will be its stability and it will act as a better leaving group.
5) Now the element which has the highest electronegativity among the given options is fluorine. Hence, the fluorine will be the good leaving group and form a stable anion after leaving. The correct order for the leaving group will be ${\text{Fe > Cl > Br > I}}$.
Therefore, the correct answer will be ${\text{ - F}}$ which shows option A as the correct choice.
Note:
The carbon atom which has halogen attached to it is electron-deficient carbon due to electronegative halogen and that is the main reason why nucleophilic attack takes place. The rate-determining step in this reaction is the attack of the nucleophile on the aromatic ring carbon-containing leaving group i.e. halogen element.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




