
Give the structural formula and IUPAC name of acetic acid. What is glacial acetic acid?
Answer
519k+ views
Hint: First draw the chemical structure and then follow the IUPAC nomenclature for naming the compound. Check whether the functional group is included in the main chain or not and then write the suffix accordingly.
Complete answer:
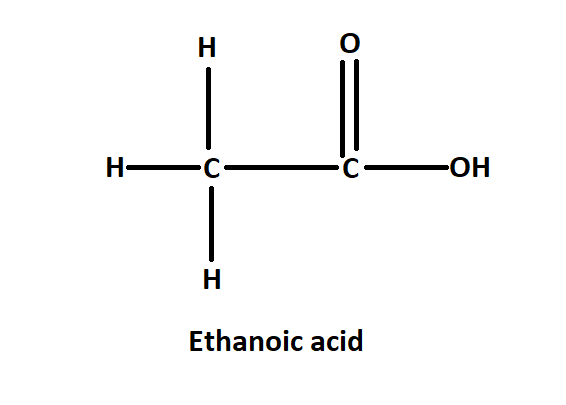
The IUPAC name of acetic acid is ethanol. It is named ethanol as it has two carbon atoms in the main chain along with one COOH group in the main chain. In acetic acid the methyl group is attached to the carbonyl functional group. The chemical formula of ethanoic acid is ${{C}_{2}}{{H}_{4}}{{O}_{2}}$. It is also written as $C{{H}_{3}}COOH$.
Acetic acid is a colourless liquid and has a pungent smell. Its molar mass is around $60.052gmo{{l}^{-1}}$. In liquid form acetic acid is a polar protic solvent.
Ethanoic acid is considered to be a weak acid but in its concentrated form it has strong corrosive power and can even damage the human skin if it comes in contact.
Acetic acid is used as an antiseptic due to its antibacterial properties. Vinegar is a solution of acetic acid in water containing $5\%$ to $20\%$ ethanoic acid by volume. Vinegar has a pungent smell and sour taste due to the presence of acetic acid in it.
Glacial acetic acid is a pure or concentrated form of acetic acid having less than $1\%$ of water content. As glacial acetic acid is concentrated, it is corrosive in nature and can cause harm to human skin if it comes in contact.
Glacial acetic acid is also known as anhydrous acetic acid as it contains a very small amount of water.
Note:
Acetic acid is formed by the fermentation process. First glucose is converted to ethanol and then ethanol is converted to ethanoic acid.
Complete answer:

The IUPAC name of acetic acid is ethanol. It is named ethanol as it has two carbon atoms in the main chain along with one COOH group in the main chain. In acetic acid the methyl group is attached to the carbonyl functional group. The chemical formula of ethanoic acid is ${{C}_{2}}{{H}_{4}}{{O}_{2}}$. It is also written as $C{{H}_{3}}COOH$.
Acetic acid is a colourless liquid and has a pungent smell. Its molar mass is around $60.052gmo{{l}^{-1}}$. In liquid form acetic acid is a polar protic solvent.
Ethanoic acid is considered to be a weak acid but in its concentrated form it has strong corrosive power and can even damage the human skin if it comes in contact.
Acetic acid is used as an antiseptic due to its antibacterial properties. Vinegar is a solution of acetic acid in water containing $5\%$ to $20\%$ ethanoic acid by volume. Vinegar has a pungent smell and sour taste due to the presence of acetic acid in it.
Glacial acetic acid is a pure or concentrated form of acetic acid having less than $1\%$ of water content. As glacial acetic acid is concentrated, it is corrosive in nature and can cause harm to human skin if it comes in contact.
Glacial acetic acid is also known as anhydrous acetic acid as it contains a very small amount of water.
Note:
Acetic acid is formed by the fermentation process. First glucose is converted to ethanol and then ethanol is converted to ethanoic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




