
Give a balanced chemical equation for the carbylamine reaction.
Answer
565.2k+ views
Hint It is also called Hofmann isocyanide synthesis. This is also used for the preparation of secondary amines.
Complete solution:
Carbylamine reaction involves the formation of isocyanides or carbylamines by the addition of aromatic and aliphatic primary amines on heating with chloroform ($\text{CHC}{{\text{l}}_{\text{3}}}$) and ethanolic potassium hydroxide. The formed carbylamine or isocyanides will have a foul smell. This reaction is called a carbylamine test. It is mainly used as a test for the primary amine as it forms carbylamine with foul smell which confirms the analyte is a primary amine.
The general equation can be given as –$\text{R-NC}$
$\text{R-N}{{\text{H}}_{\text{2}}}\text{+CHC}{{\text{l}}_{\text{3}}}\text{+3KOH}\xrightarrow{\text{heat}}\text{R-NC+3KCl+3}{{\text{H}}_{\text{2}}}\text{O}$
$\text{R-NC}$= isocyanides (carbylamine)
Aniline (an aromatic primary amine) too carries out a similar reaction. Aniline undergoing carbylamine reaction gives an isocyanide as the product.
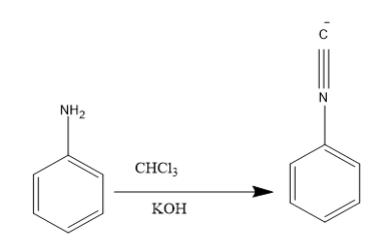
This reaction is also called Hofmann isocyanide synthesis and as it is used for the primary amine analysis test. It is also called as Saytzeff’s test.
The reaction is carried out through an intermediate formation and the intermediate formed here is dichlorocarbene. The intermediate to which the amine is added is the dehydrohalogenation product of the chloroform and the reaction proceeds through two successive steps in which the dehydrochlorination reaction takes place which yields the isocyanide.
Note: The secondary and tertiary amine doesn’t give this test as it does not form carbylamine with chloroform.
The secondary and tertiary amine will not give this test since there are more than one alkyl group which hinders the attack of$\text{-N}{{\text{H}}_{\text{2}}}$. Both the aliphatic and aromatic primary amine will show positive test results for carbylamine reaction.
Complete solution:
Carbylamine reaction involves the formation of isocyanides or carbylamines by the addition of aromatic and aliphatic primary amines on heating with chloroform ($\text{CHC}{{\text{l}}_{\text{3}}}$) and ethanolic potassium hydroxide. The formed carbylamine or isocyanides will have a foul smell. This reaction is called a carbylamine test. It is mainly used as a test for the primary amine as it forms carbylamine with foul smell which confirms the analyte is a primary amine.
The general equation can be given as –$\text{R-NC}$
$\text{R-N}{{\text{H}}_{\text{2}}}\text{+CHC}{{\text{l}}_{\text{3}}}\text{+3KOH}\xrightarrow{\text{heat}}\text{R-NC+3KCl+3}{{\text{H}}_{\text{2}}}\text{O}$
$\text{R-NC}$= isocyanides (carbylamine)
Aniline (an aromatic primary amine) too carries out a similar reaction. Aniline undergoing carbylamine reaction gives an isocyanide as the product.

This reaction is also called Hofmann isocyanide synthesis and as it is used for the primary amine analysis test. It is also called as Saytzeff’s test.
The reaction is carried out through an intermediate formation and the intermediate formed here is dichlorocarbene. The intermediate to which the amine is added is the dehydrohalogenation product of the chloroform and the reaction proceeds through two successive steps in which the dehydrochlorination reaction takes place which yields the isocyanide.
Note: The secondary and tertiary amine doesn’t give this test as it does not form carbylamine with chloroform.
The secondary and tertiary amine will not give this test since there are more than one alkyl group which hinders the attack of$\text{-N}{{\text{H}}_{\text{2}}}$. Both the aliphatic and aromatic primary amine will show positive test results for carbylamine reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




