
How many functional groups are present in the above compound?
A.2
B.3
C.4
D.5

Answer
517.5k+ views
Hint: Functional groups are defined as the groups group atoms attached to a carbon in a hydrocarbon and give their characteristic properties to the compound. Some of the examples of functional groups are alcohol group, halides, ester group, keto group, etc.
Complete answer:
Hydrocarbons are a chain of carbon compounds. These carbon compounds make either one or two bonds with their fellow carbon but sometimes some other elements are mixed in this compound so as to give their characteristic properties to the compound.
Sometimes some hydrocarbons have some alcohol group $ - OH$ and these hydrocarbons make the best of disinfectants.
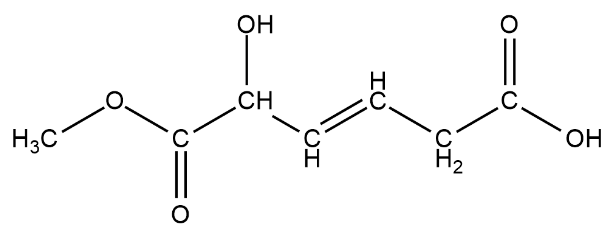
We have been here given this compound
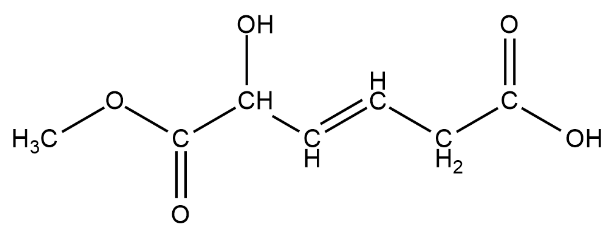 We have to find the number of functional groups in the compound
We have to find the number of functional groups in the compound
There is various type of functional groups like alkene, alcohol, aldehyde, ketone, ester, etc
There is one alcohol group present on the third carbon from left
There is one ester group $RCOOC{H_3}$ this can be seen from the left
There is also a carboxylic acid group on the second carbon from the left. The carboxylic acid looks like $ - COOH$.
The $ - OH$ group on the rightmost carbon is not an $ - OH$ functional group but a carboxylic acid group.
The total number of functional groups on this chain of hydrocarbons are three.
Note:
Functional groups also make the hydrocarbons soluble. When there is an alcoholic group attached to the hydrocarbon it increases the solubility because of the presence of a polar hydrogen bond. Now the above given compound is highly soluble because of carboxylic acid group and alcoholic group and it is also a straight chain so no hindrance.
Complete answer:
Hydrocarbons are a chain of carbon compounds. These carbon compounds make either one or two bonds with their fellow carbon but sometimes some other elements are mixed in this compound so as to give their characteristic properties to the compound.
Sometimes some hydrocarbons have some alcohol group $ - OH$ and these hydrocarbons make the best of disinfectants.
We have been here given this compound

There is various type of functional groups like alkene, alcohol, aldehyde, ketone, ester, etc
There is one alcohol group present on the third carbon from left
There is one ester group $RCOOC{H_3}$ this can be seen from the left
There is also a carboxylic acid group on the second carbon from the left. The carboxylic acid looks like $ - COOH$.
The $ - OH$ group on the rightmost carbon is not an $ - OH$ functional group but a carboxylic acid group.
The total number of functional groups on this chain of hydrocarbons are three.
Note:
Functional groups also make the hydrocarbons soluble. When there is an alcoholic group attached to the hydrocarbon it increases the solubility because of the presence of a polar hydrogen bond. Now the above given compound is highly soluble because of carboxylic acid group and alcoholic group and it is also a straight chain so no hindrance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




