
For the diazonium ions that order of reactivity towards diazo-coupling with phenol in the presence of dilute $NaOH$ is:
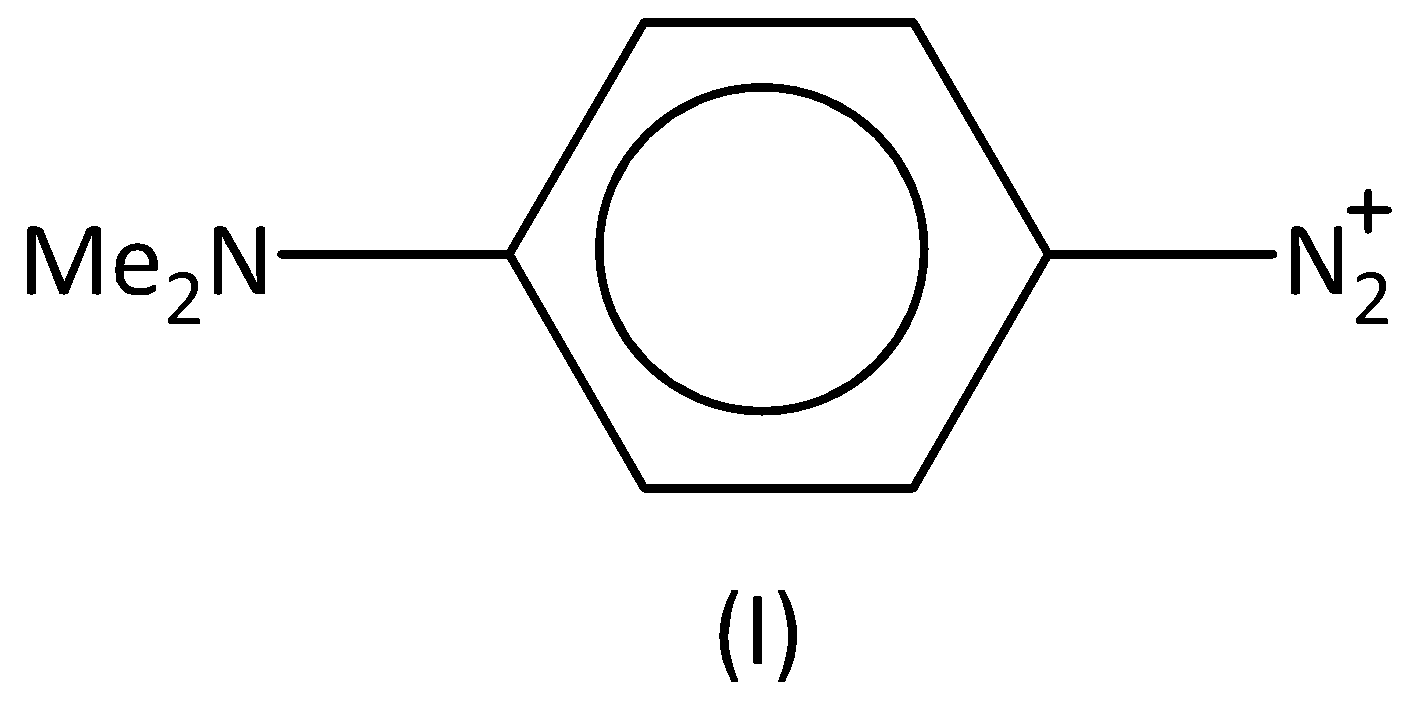
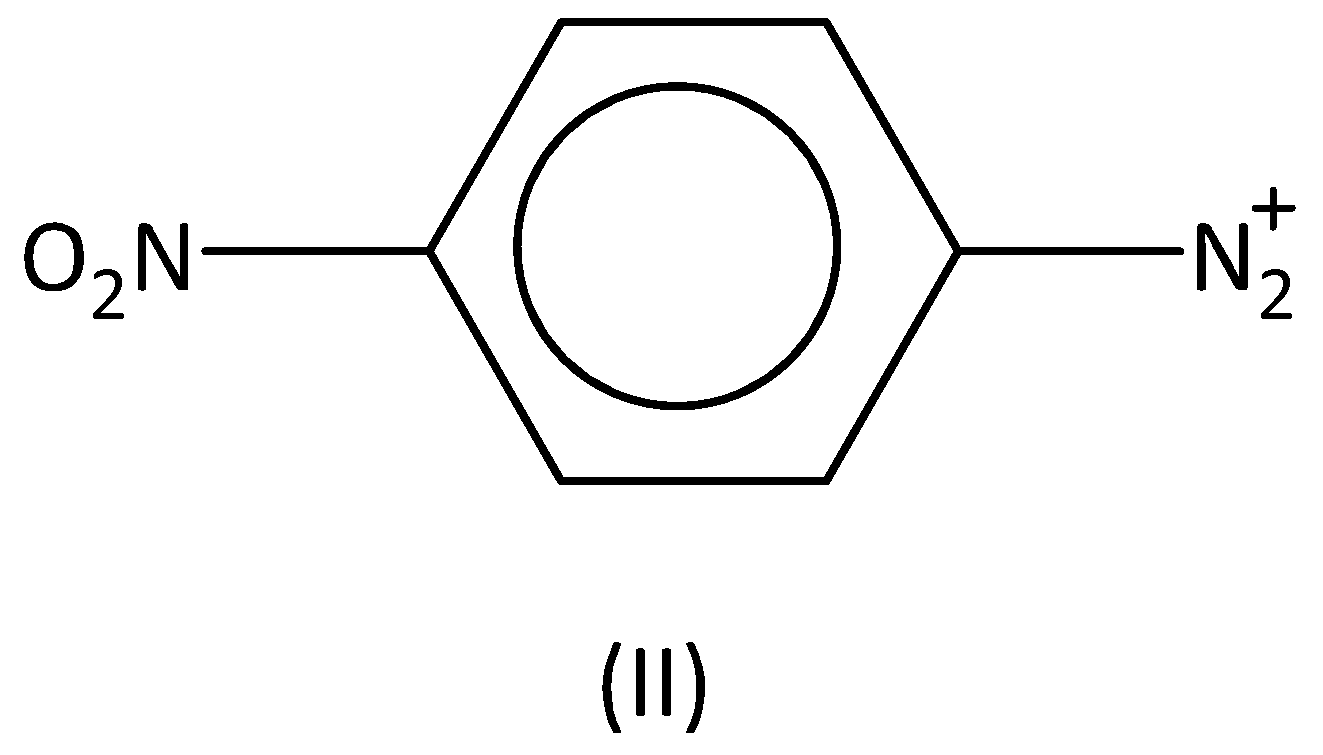
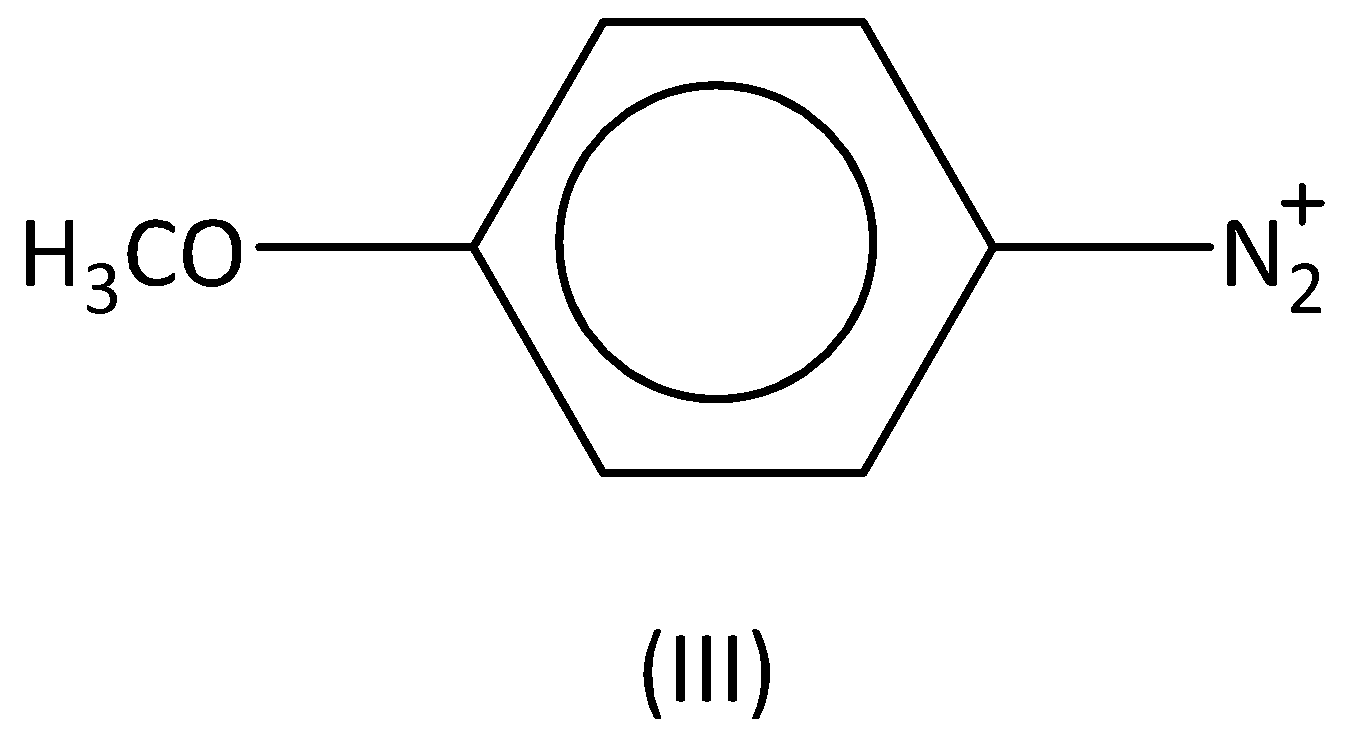
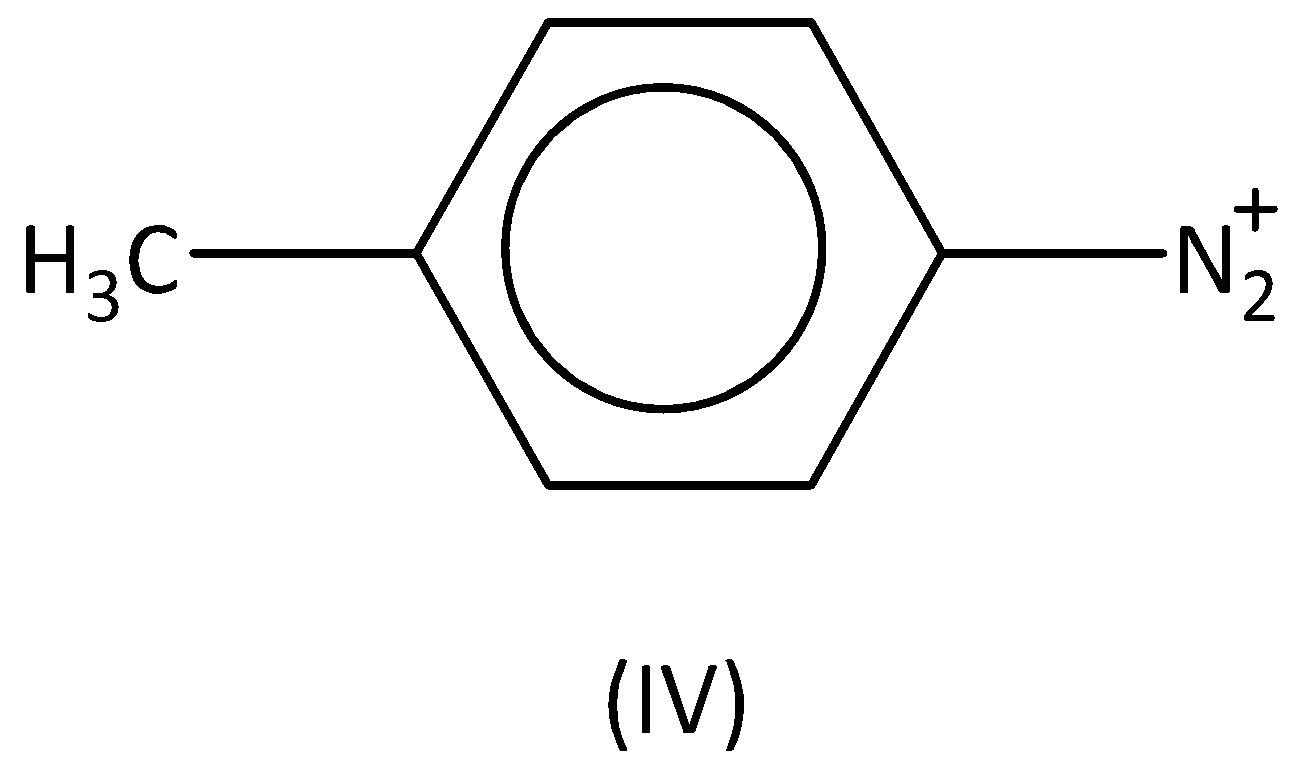
A.$I < IV < II < III$
B.$I < III < IV < II$
C.$III < I < II < IV$
D.$III < I < IV < II$




Answer
573.9k+ views
Hint:We can predict the decreasing order of reactivity of diazonium ions with the help of electron donating groups and electron releasing groups. We know that in case of diazonium ions, the functional group present in the compound leads the next incoming group to a particular position in the aromatic ring. We call this as the directive influence of the group already bonded to the benzene ring.
Complete step by step answer:
We have to remember that the electron donating groups are usually ortho/para directors for electrophilic aromatic substitutions, whereas electron withdrawing groups are usually meta directors. The halogens are ortho/para directors as they require unshared pairs of electrons to share with the aromatic ring.
We must know that the electron donating groups are referred to as activating groups, though steric effects could inhibit the reaction. An electron withdrawing group has the contradictory effect on the nucleophilicity of the ring. The electron withdrawing group takes away electron density from a \[\pi \] system, thereby making it less reactive during this kind of reaction and thus called as deactivating groups.
We have to know that the electron withdrawing group increases the electrophilicity nature of the diazonium ions whereas the electron donating groups decreases the electrophilicity nature.
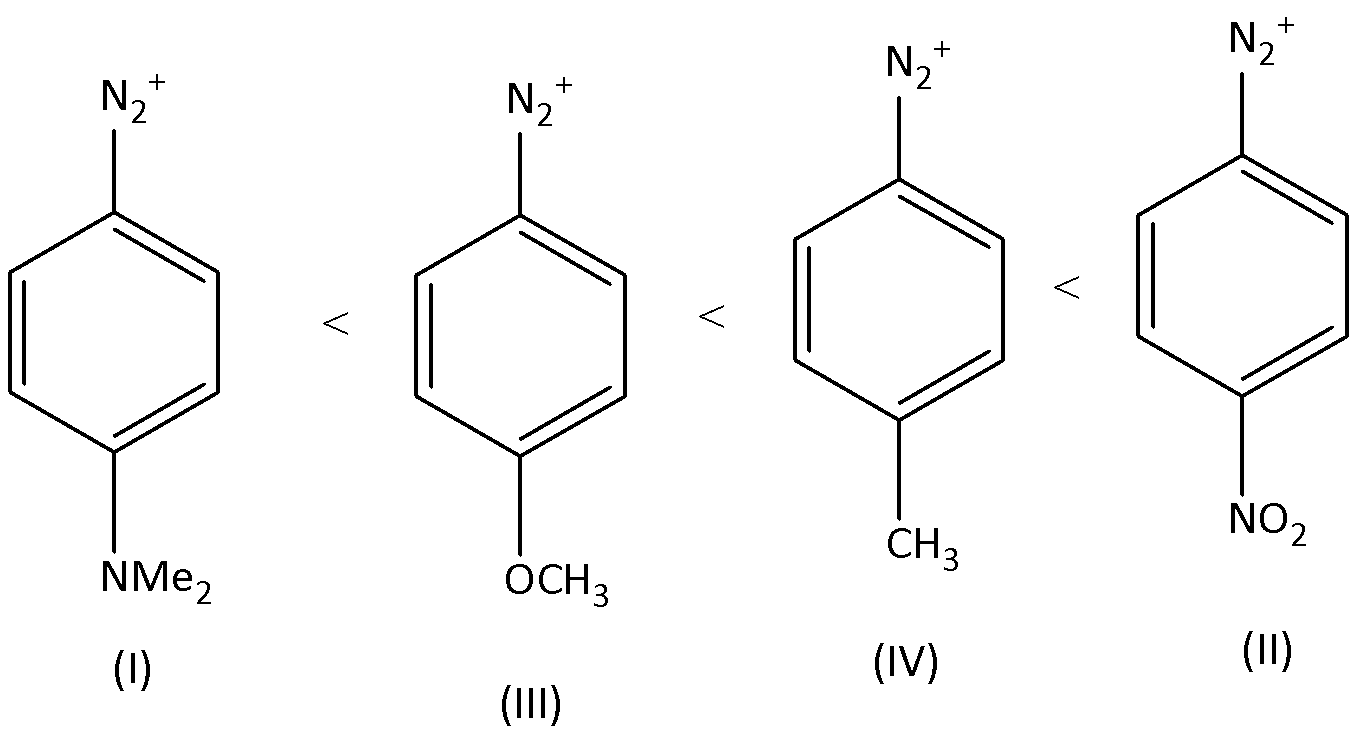
Therefore, the order of the reactivity is $I < III < IV < II$ . Option (B) is correct.
Note:
We have to remember that electron donating groups are called as activating groups and electron withdrawing groups are deactivating groups. When electron density is added to the electron donating group, the pi system tends to become more nucleophilic, and less nucleophilicity is observed when electron density is removed from the pi system by the electron withdrawing group.
Complete step by step answer:
We have to remember that the electron donating groups are usually ortho/para directors for electrophilic aromatic substitutions, whereas electron withdrawing groups are usually meta directors. The halogens are ortho/para directors as they require unshared pairs of electrons to share with the aromatic ring.
We must know that the electron donating groups are referred to as activating groups, though steric effects could inhibit the reaction. An electron withdrawing group has the contradictory effect on the nucleophilicity of the ring. The electron withdrawing group takes away electron density from a \[\pi \] system, thereby making it less reactive during this kind of reaction and thus called as deactivating groups.
We have to know that the electron withdrawing group increases the electrophilicity nature of the diazonium ions whereas the electron donating groups decreases the electrophilicity nature.

Therefore, the order of the reactivity is $I < III < IV < II$ . Option (B) is correct.
Note:
We have to remember that electron donating groups are called as activating groups and electron withdrawing groups are deactivating groups. When electron density is added to the electron donating group, the pi system tends to become more nucleophilic, and less nucleophilicity is observed when electron density is removed from the pi system by the electron withdrawing group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE




