
Fehling’s reagent gives test with:
(A) Ketones
(B) Aldehydes
(C) Alcohols
(D) Carboxyls
Answer
232.8k+ views
Hint: Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test, it is used for differentiating reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test.
Complete step by step solution:
To answer this question, we should know about the Fehling solution and for what purpose it is used.
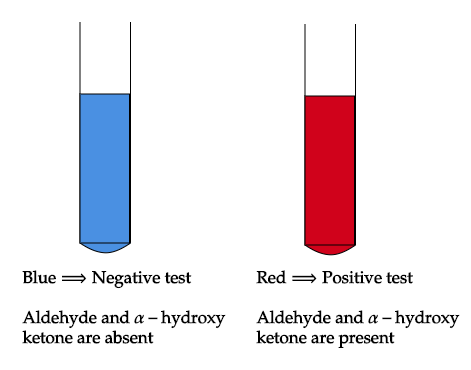
So, Fehling solution is a mixture of copper sulphate, potassium sodium tartrate, and sodium hydroxide. It is used to differentiate between water-soluble carbohydrates (aldehyde) and ketone functional groups and as a test for reducing sugar. Aldehyde undergoes this taste while ketone does not due to the presence of free electrophile which is absent in ketone.
In this test, the presence of aldehydes but not ketones are detected by reduction of the deep blue solution of copper (II) to a red precipitate of insoluble copper oxide. The test is commonly used for reducing sugars but is known to be not specific for aldehydes. For example, fructose gives a positive test with Fehling's solution as does acetoin (exception).
Let’s see how aldehyde gives a positive test to Fehling’s test.
So, taking an example:
Acetaldehyde reacts with Fehling’s solution and gives acetic acid as the main product. The reaction is indicated by the red precipitation of copper oxide.
$C{{H}_{3}}-CHO+2C{{u}^{++}}+4O{{H}^{-}}\xrightarrow{Heat}C{{H}_{3}}COOH+C{{u}_{2}}O+3{{H}_{2}}O$ So, looking at the options except for aldehydes, other options do not have a free alpha-hydroxy carbonyl group or an electrophilic group.
Hence, the correct option is B.
Additional information:
Method of preparation of Fehling solution:
Fehling solution is a mixture of two solutions, Fehling's "A" and Fehling's "B". Fehling's "A" uses 7 g \[CuS{{O}_{4}}.5{{H}_{2}}O\] dissolved in distilled water containing 2 drops of dilute sulfuric acid. Fehling's "B" uses 35g of potassium tartrate and 12g of \[NaOH\] in 100 ml of distilled water. These two solutions should be stoppered and stored until needed.
For the test:
- Mix 15 ml of the solution-"A" with 15 ml of the solution-"B"
- Add 2 ml of this mixture to an empty test tube.
- Add 3 drops of the compound to be tested to the tube.
- Place the tube in a water-bath at \[60{}^\circ \] C.
A positive test is indicated by a green suspension and a red precipitate. The test is sensitive enough that even 1 mg of glucose will produce the characteristic red colour of the compound.
Note: The possible mistake could be, you may be confused with option A i.e. ketones, but ketones do not undergo reduction giving deep blue solution when reacted with Fehling solution due to less electrophilicity of carbonyl carbon (alpha-hydroxy ketones). So, Fehling’s test is used to differentiate between reducing sugar and non-reducing sugar.
Complete step by step solution:
To answer this question, we should know about the Fehling solution and for what purpose it is used.
So, Fehling solution is a mixture of copper sulphate, potassium sodium tartrate, and sodium hydroxide. It is used to differentiate between water-soluble carbohydrates (aldehyde) and ketone functional groups and as a test for reducing sugar. Aldehyde undergoes this taste while ketone does not due to the presence of free electrophile which is absent in ketone.
In this test, the presence of aldehydes but not ketones are detected by reduction of the deep blue solution of copper (II) to a red precipitate of insoluble copper oxide. The test is commonly used for reducing sugars but is known to be not specific for aldehydes. For example, fructose gives a positive test with Fehling's solution as does acetoin (exception).
Let’s see how aldehyde gives a positive test to Fehling’s test.
So, taking an example:
Acetaldehyde reacts with Fehling’s solution and gives acetic acid as the main product. The reaction is indicated by the red precipitation of copper oxide.

$C{{H}_{3}}-CHO+2C{{u}^{++}}+4O{{H}^{-}}\xrightarrow{Heat}C{{H}_{3}}COOH+C{{u}_{2}}O+3{{H}_{2}}O$ So, looking at the options except for aldehydes, other options do not have a free alpha-hydroxy carbonyl group or an electrophilic group.
Hence, the correct option is B.
Additional information:
Method of preparation of Fehling solution:
Fehling solution is a mixture of two solutions, Fehling's "A" and Fehling's "B". Fehling's "A" uses 7 g \[CuS{{O}_{4}}.5{{H}_{2}}O\] dissolved in distilled water containing 2 drops of dilute sulfuric acid. Fehling's "B" uses 35g of potassium tartrate and 12g of \[NaOH\] in 100 ml of distilled water. These two solutions should be stoppered and stored until needed.
For the test:
- Mix 15 ml of the solution-"A" with 15 ml of the solution-"B"
- Add 2 ml of this mixture to an empty test tube.
- Add 3 drops of the compound to be tested to the tube.
- Place the tube in a water-bath at \[60{}^\circ \] C.
A positive test is indicated by a green suspension and a red precipitate. The test is sensitive enough that even 1 mg of glucose will produce the characteristic red colour of the compound.
Note: The possible mistake could be, you may be confused with option A i.e. ketones, but ketones do not undergo reduction giving deep blue solution when reacted with Fehling solution due to less electrophilicity of carbonyl carbon (alpha-hydroxy ketones). So, Fehling’s test is used to differentiate between reducing sugar and non-reducing sugar.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




