
Explain $S{N_1} $reaction with an appropriate reaction.
Answer
561.6k+ views
Hint: This reaction is a nucleophilic substitution reaction which means that the functional group already present in the compound is replaced by another functional group that is also a nucleophile. A nucleophile is an electron rich species. This reaction takes place in polar solvent.
Complete step by step answer:
The $S{N_1} $ reaction is a two-step reaction that involves the substitution of a nucleophile in the presence of a polar solution. This reaction depends on the concentration of substrate that is being used. On increasing the concentration of substrate, the rate of the reaction also increases. The reaction does not depend on the concentration of the nucleophile.

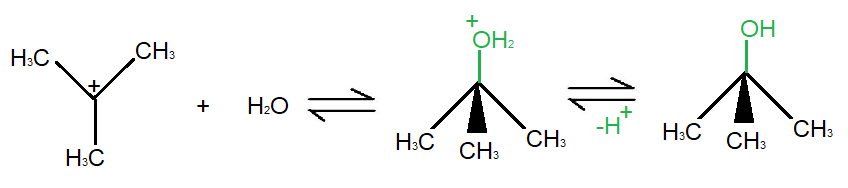
As seen in the diagram the first step of the reaction consists of the ionization of the existing nucleophile in the presence of a polar protic solvent. This is the slow step of the reaction and therefore, it is the rate defining step. This step results in the formation of carbocation.
The second step is the attack of nucleophiles on the carbocation. This results in the attachment of the electron rich nucleophile onto the electron deficient electrophile. This attachment can happen in two ways. The first possibility is if the reaction occurs by the attachment of the nucleophile at the same site as that of the previous nucleophile. This leads to the formation of a product that has identical geometry as that of the reactant but contains a different functional group.
The second possibility is if the nucleophile is attached at a position that is opposite to the site of the previous functional group. This means that the attack will be at the backside of the carbocation. Resulting in the formation of a compound that is inverted with respect to position of the functional group.
This leads to the formation of two substances called enantiomers. Enantiomers can be defined as two compounds that are non-superimposable mirror images of each other. The concentration of both forms is usually equal therefore, we can say that the product formed is a racemic mixture.
Note: $S{N_1} $ reaction forms two products that are enantiomers of each other. the products together form a racemic mixture.
This reaction uses polar protic solvent. Protic means that the compound used contains hydrogen bonds for example ${H_2} O, C{H_3} OH$ and others.
Complete step by step answer:
The $S{N_1} $ reaction is a two-step reaction that involves the substitution of a nucleophile in the presence of a polar solution. This reaction depends on the concentration of substrate that is being used. On increasing the concentration of substrate, the rate of the reaction also increases. The reaction does not depend on the concentration of the nucleophile.


As seen in the diagram the first step of the reaction consists of the ionization of the existing nucleophile in the presence of a polar protic solvent. This is the slow step of the reaction and therefore, it is the rate defining step. This step results in the formation of carbocation.
The second step is the attack of nucleophiles on the carbocation. This results in the attachment of the electron rich nucleophile onto the electron deficient electrophile. This attachment can happen in two ways. The first possibility is if the reaction occurs by the attachment of the nucleophile at the same site as that of the previous nucleophile. This leads to the formation of a product that has identical geometry as that of the reactant but contains a different functional group.
The second possibility is if the nucleophile is attached at a position that is opposite to the site of the previous functional group. This means that the attack will be at the backside of the carbocation. Resulting in the formation of a compound that is inverted with respect to position of the functional group.
This leads to the formation of two substances called enantiomers. Enantiomers can be defined as two compounds that are non-superimposable mirror images of each other. The concentration of both forms is usually equal therefore, we can say that the product formed is a racemic mixture.
Note: $S{N_1} $ reaction forms two products that are enantiomers of each other. the products together form a racemic mixture.
This reaction uses polar protic solvent. Protic means that the compound used contains hydrogen bonds for example ${H_2} O, C{H_3} OH$ and others.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




