
Dress material Terylene is essentially:
A) A nylon product
B) Acrylic product
C) Polyester product
D) Rayon product
Answer
552.9k+ views
Hint:Terylene is an artificial synthetic fiber extensively used in the textile industry to make hard wear clothes like sarees, tapestry, and dress material. It is mixed with natural fiber (like wool and cotton) to make huge varieties of clothes.
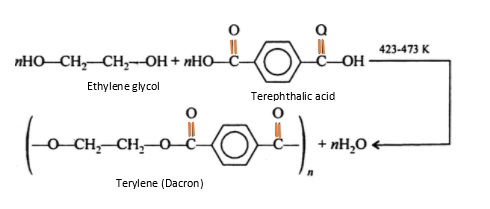
Complete step by step answer:1) Dress material terylene is essentially a polyester product; made by condensation polymerization ethylene glycol \[\left( {{\text{ethane - 1,2 - diol}}} \right)\]and terephthalic acid \[\left( {{\text{benzene - 1,4 - dicarboxylic acid}}} \right)\]at \[\left( {{\text{benzene - 1,4 - dicarboxylic acid}}} \right)\], which is obtained from petroleum. Since, it is obtained by condensation polymerization, in which the molecular mass of a polymer is not a whole number of monomers due to loss of water molecules, that’s why it is also called step-growth polymer.
2) Terylene possesses qualities such as lightness, toughness, resistance to scraping and to sunlight, and durability (particularly in the form of staple fiber). It was discovered by J. R. Whinfield and J. T. Dickson, in March \[{\text{1941}}\].
3) It is not affected by acidic substances, neither bleaches nor dry cleaning agents can destroy it and crease resist too. So, it is also used to prepare the seat belt, sails, and tire cords.
Hence, option (C) is the correct choice.
Note:
Terylene is the first polyester fabric ever produced. It may also be known by the brand names Dacron in the US, Lavan in Russia and the former Soviet Union, and Terylene in the UK. It can be easy in the wash and dries quickly, so it is used for the manufacture of wash and wear fabrics and mixed with cotton and wool to increase their resistance to wear and tear.
Complete step by step answer:1) Dress material terylene is essentially a polyester product; made by condensation polymerization ethylene glycol \[\left( {{\text{ethane - 1,2 - diol}}} \right)\]and terephthalic acid \[\left( {{\text{benzene - 1,4 - dicarboxylic acid}}} \right)\]at \[\left( {{\text{benzene - 1,4 - dicarboxylic acid}}} \right)\], which is obtained from petroleum. Since, it is obtained by condensation polymerization, in which the molecular mass of a polymer is not a whole number of monomers due to loss of water molecules, that’s why it is also called step-growth polymer.

2) Terylene possesses qualities such as lightness, toughness, resistance to scraping and to sunlight, and durability (particularly in the form of staple fiber). It was discovered by J. R. Whinfield and J. T. Dickson, in March \[{\text{1941}}\].
3) It is not affected by acidic substances, neither bleaches nor dry cleaning agents can destroy it and crease resist too. So, it is also used to prepare the seat belt, sails, and tire cords.
Hence, option (C) is the correct choice.
Note:
Terylene is the first polyester fabric ever produced. It may also be known by the brand names Dacron in the US, Lavan in Russia and the former Soviet Union, and Terylene in the UK. It can be easy in the wash and dries quickly, so it is used for the manufacture of wash and wear fabrics and mixed with cotton and wool to increase their resistance to wear and tear.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




