
Draw the structure of urotropine and write its use.
Answer
552.9k+ views
Hint:Urotropine is a heterocyclic organic compound also known as Methenamine or Hexamethylenetetramine. One can write its use for medicinal purposes and in industries with specifics. Also, this compound has some fuel properties which can be explained.
Complete step by step answer:1)First we will discuss the Urotropine which is a white crystalline granular compound with a formula \[{\left( {C{H_2}} \right)_6}{N_4}\] that is highly soluble in water and polar organic solvents. It is prepared by the condensation reaction of formaldehyde and ammonia.
\[6HCHO{\text{ }} + 4N{H_3}\;\;\; \to \;\;{\left( {C{H_2}} \right)_6}{N_4}\; + \;6{H_2}O\]
2) It has a cage-like structure which is similar to adamantine. The structure of Urotropine is as below,
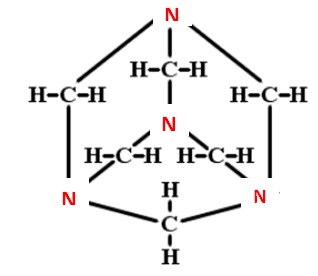
3) Now let us see the uses of urotropine as Methenamine is typically used to treat recurrence of infection and in the salt from it is used to treat chronic urinary tract infection as the mandelic salt. In a form of cream or spray, it is used to treat concomitant odor and excessive sweating. Its anti-infective property is due to the slow release of formaldehyde \[\left( {C{H_2}O} \right)\] as it kills bacteria (bactericidal) by hydrolysis at the acidic pH of ${\text{0}} \cdot {\text{2}}$ molars.
4) It is used in a variety of industrial applications which includes the preparation of powdery or liquid phenolic resins (Novolac resins) and its molding compounds where it is added as a hardening component. It is used as chemical intermediate/chemical synthesis as the binder in the clutch lining, corrosion inhibitor for steel, and also used as rubber linker due to which it prevents rubber vulcanization.
5) Hexamethylenetetramine with \[{\text{1,3,5 - trioxane}}\], also used as a solid fuel as it burns smokeless, has a high energy density of \[{\text{30MJ/Kg}}\], leaves no ashes, and does not liquefy while burning. Also used by fire protection laboratories to test the flammability of carpets and rugs.
Note:
Aleksandr Butlerov in \[{\text{1859}}\] discovered Hexamethylenetetramine. It is approved by the EU in the usage of food preservatives/additives too. It is also used as a versatile reagent in organic chemistry and as the basic ingredient in making RDX.
Complete step by step answer:1)First we will discuss the Urotropine which is a white crystalline granular compound with a formula \[{\left( {C{H_2}} \right)_6}{N_4}\] that is highly soluble in water and polar organic solvents. It is prepared by the condensation reaction of formaldehyde and ammonia.
\[6HCHO{\text{ }} + 4N{H_3}\;\;\; \to \;\;{\left( {C{H_2}} \right)_6}{N_4}\; + \;6{H_2}O\]
2) It has a cage-like structure which is similar to adamantine. The structure of Urotropine is as below,

3) Now let us see the uses of urotropine as Methenamine is typically used to treat recurrence of infection and in the salt from it is used to treat chronic urinary tract infection as the mandelic salt. In a form of cream or spray, it is used to treat concomitant odor and excessive sweating. Its anti-infective property is due to the slow release of formaldehyde \[\left( {C{H_2}O} \right)\] as it kills bacteria (bactericidal) by hydrolysis at the acidic pH of ${\text{0}} \cdot {\text{2}}$ molars.
4) It is used in a variety of industrial applications which includes the preparation of powdery or liquid phenolic resins (Novolac resins) and its molding compounds where it is added as a hardening component. It is used as chemical intermediate/chemical synthesis as the binder in the clutch lining, corrosion inhibitor for steel, and also used as rubber linker due to which it prevents rubber vulcanization.
5) Hexamethylenetetramine with \[{\text{1,3,5 - trioxane}}\], also used as a solid fuel as it burns smokeless, has a high energy density of \[{\text{30MJ/Kg}}\], leaves no ashes, and does not liquefy while burning. Also used by fire protection laboratories to test the flammability of carpets and rugs.
Note:
Aleksandr Butlerov in \[{\text{1859}}\] discovered Hexamethylenetetramine. It is approved by the EU in the usage of food preservatives/additives too. It is also used as a versatile reagent in organic chemistry and as the basic ingredient in making RDX.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




