
Draw the structure of ${{N}_{2}}{{O}_{3}}$ and ${{N}_{2}}{{O}_{5}}$ .
Answer
592.5k+ views
Hint: First calculate the valence electrons of each atom present in compounds given then we can draw the Lewis structure of given compounds. Nitrogen and oxygen have 5 and 6 valence electrons respectively.
Complete step by step answer:
- Lewis structure is a graphic representation of the electron distribution around atoms. When we draw the Lewis structures, it helps us to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
- To draw structure of ${{N}_{2}}{{O}_{3}}$ and ${{N}_{2}}{{O}_{5}}$ , we will first calculate number of valence electrons in the compound.
We should first find the total number of valence electrons. As in both compounds Nitrogen and oxygen atoms are present, so will calculate at same time. Note that nitrogen and oxygen have 5 and 6 valence electrons respectively.
${{N}_{2}}{{O}_{3}}$ =$(5\times 2)+(6\times 3)=28$ electrons
${{N}_{2}}{{O}_{5}}$ =\[(5\times 2)+(6\times 5)=40\] electrons
- We should follow the octet rule in filling electrons at the outer orbital. So, we will form covalent bonds between the two atoms which are formed by sharing one electron from each atom.
The structure of ${{N}_{2}}{{O}_{3}}$ will look like:
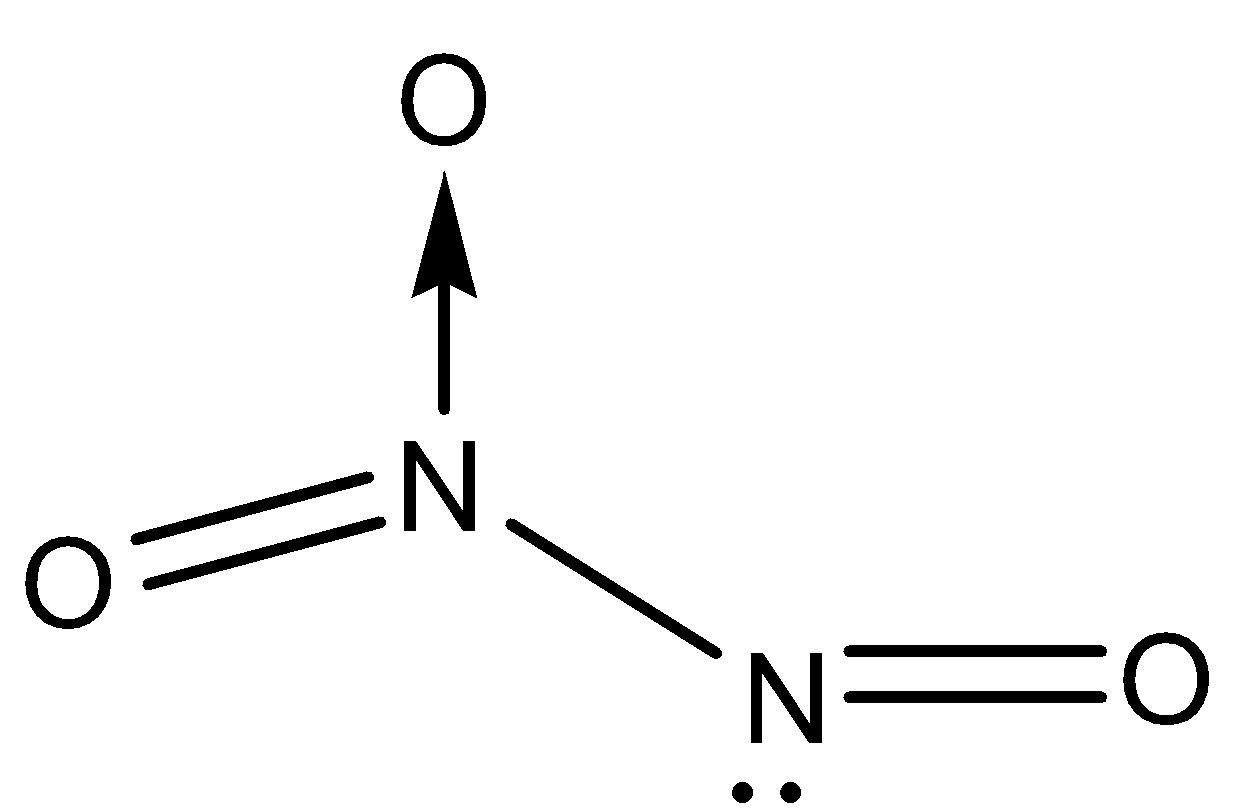
- Here, we can see a bond that is shown by an arrow between nitrogen and oxygen. This shows a covalent coordinate bond which is formed by donation of an electron pair from nitrogen to oxygen. Thus, we can conclude that ${{N}_{2}}{{O}_{3}}$ contains a total of 6 bonds in which one is covalent coordinate bond, two are $\pi $ bonds and three are $\sigma $ covalent bonds.
-The structure of ${{N}_{2}}{{O}_{5}}$ will look like:
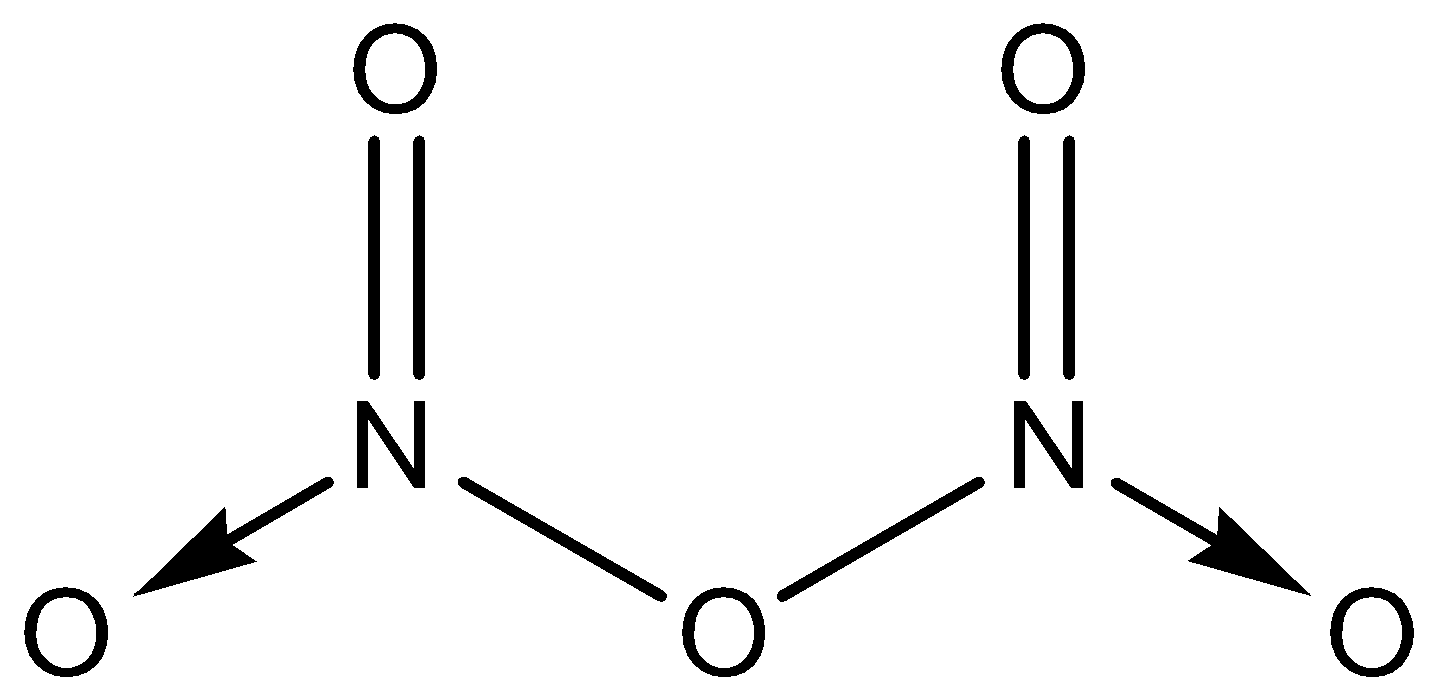
- Here, in the structure of ${{N}_{2}}{{O}_{5}}$ , we can see that there are a total 8 bonds present. Two of them are covalent coordinate bonds. Two are $\pi $ bonds and four $\sigma $ bonds are present in the molecule.
Note:
- ${{N}_{2}}{{O}_{3}}$ or Dinitrogen trioxide appears as a blue liquid with a sharp, unpleasant chemical odour. It is used in special purpose fuels. It is a strong irritant to skin, eyes and mucous membranes.
- Dinitrogen Pentoxide (${{N}_{2}}{{O}_{5}}$ ) used in solvents that are not based on water, so that molecules that are very sensitive to water can be easily nitrated. We used it as a nitrating agent in modern synthetic organic chemistry. A mixture of ${{N}_{2}}{{O}_{5}}$ and $HN{{O}_{3}}$ is also a good and strong nitrating agent.
Complete step by step answer:
- Lewis structure is a graphic representation of the electron distribution around atoms. When we draw the Lewis structures, it helps us to predict the number and type of bonds that may be formed around an atom. A Lewis structure also helps to make a prediction about the geometry of a molecule.
- To draw structure of ${{N}_{2}}{{O}_{3}}$ and ${{N}_{2}}{{O}_{5}}$ , we will first calculate number of valence electrons in the compound.
We should first find the total number of valence electrons. As in both compounds Nitrogen and oxygen atoms are present, so will calculate at same time. Note that nitrogen and oxygen have 5 and 6 valence electrons respectively.
${{N}_{2}}{{O}_{3}}$ =$(5\times 2)+(6\times 3)=28$ electrons
${{N}_{2}}{{O}_{5}}$ =\[(5\times 2)+(6\times 5)=40\] electrons
- We should follow the octet rule in filling electrons at the outer orbital. So, we will form covalent bonds between the two atoms which are formed by sharing one electron from each atom.
The structure of ${{N}_{2}}{{O}_{3}}$ will look like:

- Here, we can see a bond that is shown by an arrow between nitrogen and oxygen. This shows a covalent coordinate bond which is formed by donation of an electron pair from nitrogen to oxygen. Thus, we can conclude that ${{N}_{2}}{{O}_{3}}$ contains a total of 6 bonds in which one is covalent coordinate bond, two are $\pi $ bonds and three are $\sigma $ covalent bonds.
-The structure of ${{N}_{2}}{{O}_{5}}$ will look like:

- Here, in the structure of ${{N}_{2}}{{O}_{5}}$ , we can see that there are a total 8 bonds present. Two of them are covalent coordinate bonds. Two are $\pi $ bonds and four $\sigma $ bonds are present in the molecule.
Note:
- ${{N}_{2}}{{O}_{3}}$ or Dinitrogen trioxide appears as a blue liquid with a sharp, unpleasant chemical odour. It is used in special purpose fuels. It is a strong irritant to skin, eyes and mucous membranes.
- Dinitrogen Pentoxide (${{N}_{2}}{{O}_{5}}$ ) used in solvents that are not based on water, so that molecules that are very sensitive to water can be easily nitrated. We used it as a nitrating agent in modern synthetic organic chemistry. A mixture of ${{N}_{2}}{{O}_{5}}$ and $HN{{O}_{3}}$ is also a good and strong nitrating agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




