
Draw the structure of acetonitrile.
Answer
565.2k+ views
Hint:. Acetonitrile is also known as methyl cyanide. It is a chemical compound with the molecular formula \[C{H_3}C \equiv N\]. We know that carbon will be the central metal atom and Nitrogen and the other carbon atom with methyl group will be attached to it.
Complete step by step answer:
Let’s first see what are the elements present in the acetonitrile. Acetonitrile is actually the IUPAC name for methyl cyanide. Acetonitrile is having a formula of \[C{H_3}C \equiv N\]. From the formula we can see that there are three elements present in the carbon atom. They are C, N and H atoms. There are two carbon atoms present in the acetonitrile.
- Carbon will be the central atom in this compound. Carbon is having a valency of 4. It can form only four bonds, whereas nitrogen will have a valency of 3. Since nitrogen is not bonded to any other atom, all the three-valence electrons will be forming a triple bond with the three-valence electron of the carbon atom. Remaining there will be one valence electron in the central carbon atom, it will be forming a single bond with the other carbon atom. Now the other carbon is left with three valence electrons. These electrons will be forming a single bond with three hydrogen with one valence electron. This leads to the formation of methyl groups.
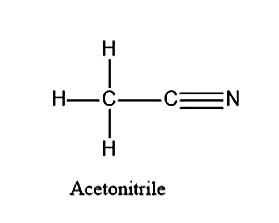
Additional information: Acetonitrile have the following characteristics.
- Acetonitrile is a transparent liquid.
- It has an aromatic odour.
- It is a polar aprotic solvent.
- There are other names for acetonitrile, they are ethyl nitrile, methyl cyanide and ethane nitrile.
- Acetonitrile has various applications such as, it is used in perfumes, pharmaceuticals, nail polish removers, pesticides and rubber products.
Note: The carbon which is attached to the nitrogen group will have \[sp\] hybridization because it is attached to only two atoms namely nitrogen and the carbon of the methyl group. The methyl group carbon will be having a hybridization of \[s{p^3}\] because it has been attached to 4 atoms namely, 3 hydrogen atoms and one carbon atom.
Complete step by step answer:
Let’s first see what are the elements present in the acetonitrile. Acetonitrile is actually the IUPAC name for methyl cyanide. Acetonitrile is having a formula of \[C{H_3}C \equiv N\]. From the formula we can see that there are three elements present in the carbon atom. They are C, N and H atoms. There are two carbon atoms present in the acetonitrile.
- Carbon will be the central atom in this compound. Carbon is having a valency of 4. It can form only four bonds, whereas nitrogen will have a valency of 3. Since nitrogen is not bonded to any other atom, all the three-valence electrons will be forming a triple bond with the three-valence electron of the carbon atom. Remaining there will be one valence electron in the central carbon atom, it will be forming a single bond with the other carbon atom. Now the other carbon is left with three valence electrons. These electrons will be forming a single bond with three hydrogen with one valence electron. This leads to the formation of methyl groups.

Additional information: Acetonitrile have the following characteristics.
- Acetonitrile is a transparent liquid.
- It has an aromatic odour.
- It is a polar aprotic solvent.
- There are other names for acetonitrile, they are ethyl nitrile, methyl cyanide and ethane nitrile.
- Acetonitrile has various applications such as, it is used in perfumes, pharmaceuticals, nail polish removers, pesticides and rubber products.
Note: The carbon which is attached to the nitrogen group will have \[sp\] hybridization because it is attached to only two atoms namely nitrogen and the carbon of the methyl group. The methyl group carbon will be having a hybridization of \[s{p^3}\] because it has been attached to 4 atoms namely, 3 hydrogen atoms and one carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




