
Draw structure of all the aldehydes with the formula ${{C}_{5}}{{H}_{10}}O$.
Answer
593.7k+ views
Hint: An aldehyde is an organic compound which has a –CHO functional group. To draw all the structures, we can start by drawing a linear chain with the substituents and then drawing branched carbon chains at non-equivalent carbon atoms.
Complete Step by Step Solution: We know that aldehydes are organic compounds and they contain a –CHO functional group. It consists of a carbonyl centre, which means a carbon will be double bonded to an oxygen atom and the same carbon centre is attached to the alkyl group or other hydrogen atoms.
To draw all the aldehydes with the given formula, we will keep a carbonyl centre fixed and change the alkyl groups attached to it.
If we remove the –CHO portion from the formula, we are left with ${{C}_{4}}{{H}_{9}}$, a butyl group as it contains four carbon atoms.
For a butyl group, we can have four possible isomers. Therefore, there will be four possible structures of aldehyde.
We will start by arranging all the carbons linearly and putting the aldehyde group at the end of the chain, which will give us the structure-
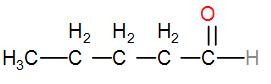
The name of this compound is pentanal.
Next, instead of placing the carbon atoms linearly, we can form a branched chain and it will give us the structure-
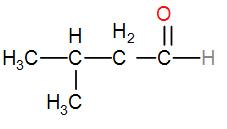
The name of this chain is 3-methylbutanal as the methyl is attached to the third carbon with respect to the aldehyde functional group.
Next, we can place the methyl group at the 2-position with respect to the functional group and it will give us the structure-
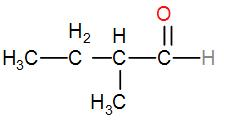
The name of this compound is 2-methylbutanal.
And lastly, we can take a single carbon atom and attach all the groups to it which will give us the structure-
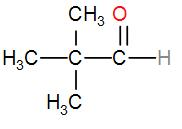
And the name of this compound is 2,2-dimethylpropanal.
Therefore, these are the possible structures of aldehydes of the formula${{C}_{5}}{{H}_{10}}O$.
Note: We can also draw ketones from the same formula which has a –CO functional group fixed therefore, we fixed the position of the aldehyde first and then rearranged the other groups or else it would have been very confusing. The structures obtained here are isomers as they have the same chemical formula.
Complete Step by Step Solution: We know that aldehydes are organic compounds and they contain a –CHO functional group. It consists of a carbonyl centre, which means a carbon will be double bonded to an oxygen atom and the same carbon centre is attached to the alkyl group or other hydrogen atoms.
To draw all the aldehydes with the given formula, we will keep a carbonyl centre fixed and change the alkyl groups attached to it.
If we remove the –CHO portion from the formula, we are left with ${{C}_{4}}{{H}_{9}}$, a butyl group as it contains four carbon atoms.
For a butyl group, we can have four possible isomers. Therefore, there will be four possible structures of aldehyde.
We will start by arranging all the carbons linearly and putting the aldehyde group at the end of the chain, which will give us the structure-
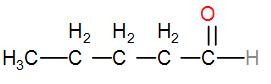
The name of this compound is pentanal.
Next, instead of placing the carbon atoms linearly, we can form a branched chain and it will give us the structure-

The name of this chain is 3-methylbutanal as the methyl is attached to the third carbon with respect to the aldehyde functional group.
Next, we can place the methyl group at the 2-position with respect to the functional group and it will give us the structure-

The name of this compound is 2-methylbutanal.
And lastly, we can take a single carbon atom and attach all the groups to it which will give us the structure-

And the name of this compound is 2,2-dimethylpropanal.
Therefore, these are the possible structures of aldehydes of the formula${{C}_{5}}{{H}_{10}}O$.
Note: We can also draw ketones from the same formula which has a –CO functional group fixed therefore, we fixed the position of the aldehyde first and then rearranged the other groups or else it would have been very confusing. The structures obtained here are isomers as they have the same chemical formula.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




