
How would you draw all the resonance structures for nitrate, $NO_{3}^{-}$ ?
Answer
565.5k+ views
Hint: As we know that resonance is the process of de-localization of the $\pi $- electrons that takes place in any compound which basically causes lowering of the energy of the compound and hence providing stability to it. And the energy which is released from the compound when it undergoes resonance is called resonance energy.
Complete Solution :
- It is found that in resonance a molecule can be expressed in different forms, and none of them can explain all the properties of the molecule. The main structure of the molecule is called a resonance hybrid.
- As we know that resonance is the extension of the fact that we can describe the bonding between the atoms in a chemical compound with the help of a Lewis structure.
- It is found that in some cases we can draw more than one Lewis structure, and the experimental properties are inconsistent with any one structure.
- In such a type of situation, some contributing structures are considered together as an average, and the molecule is represented by a resonance hybrid. It is found that in this several Lewis structures are used to describe the true structure.
- Nitrogen is the central atom in $NO_{3}^{-}$, which is singly bonded to one oxygen atom. The oxygen atoms hold a charge of -1. It is found that the central atom has a charge of +1, and the overall charge on the central atom is -1.
- It is found that there are three resonance structures possible for $NO_{3}^{-}$, these are:
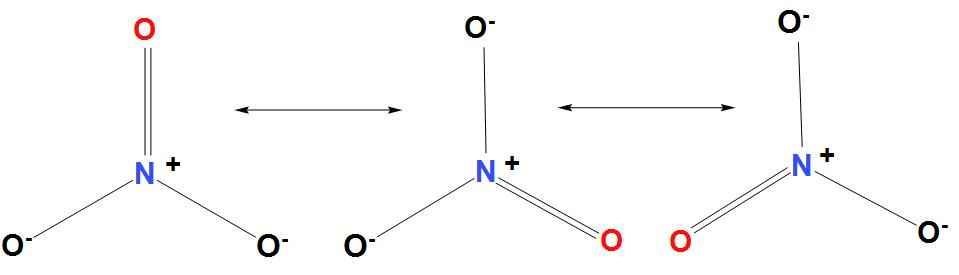
- Hence we can conclude that three resonance structures are there for $NO_{3}^{-}$.
Note: As we know that resonance hybrid represents the main molecule as the average of the contributing structures. Resonance is basically a hypothetical phenomenon; it is actually impossible to say whether any molecule with a resonance structure is in one or another configuration.
Complete Solution :
- It is found that in resonance a molecule can be expressed in different forms, and none of them can explain all the properties of the molecule. The main structure of the molecule is called a resonance hybrid.
- As we know that resonance is the extension of the fact that we can describe the bonding between the atoms in a chemical compound with the help of a Lewis structure.
- It is found that in some cases we can draw more than one Lewis structure, and the experimental properties are inconsistent with any one structure.
- In such a type of situation, some contributing structures are considered together as an average, and the molecule is represented by a resonance hybrid. It is found that in this several Lewis structures are used to describe the true structure.
- Nitrogen is the central atom in $NO_{3}^{-}$, which is singly bonded to one oxygen atom. The oxygen atoms hold a charge of -1. It is found that the central atom has a charge of +1, and the overall charge on the central atom is -1.
- It is found that there are three resonance structures possible for $NO_{3}^{-}$, these are:

- Hence we can conclude that three resonance structures are there for $NO_{3}^{-}$.
Note: As we know that resonance hybrid represents the main molecule as the average of the contributing structures. Resonance is basically a hypothetical phenomenon; it is actually impossible to say whether any molecule with a resonance structure is in one or another configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




