
D-glucose and D-fructose can be differentiated by
A. Fehling's solution
B. Tollens reagent
C. Benedict test
D. \[{\text{B}}{{\text{r}}_2}{\text{/}}{{\text{H}}_2}{\text{O}}\]
Answer
582.9k+ views
Hint: Both D-glucose and D-fructose are simple monosaccharides. They are carbohydrates. D-glucose is a poly-hydroxy aldehyde and D-fructose is a poly-hydroxy ketone.
Complete step by step answer:
> Select the reagent that can react with either glucose or fructose. The reagent should not react with both. Also during the selected reagent with base, inter-conversion of glucose and fructose should not happen.
> Benedict's test is used to tell if the sugar is reducing or not. When simple carbohydrates have free aldehyde or ketone functional groups, they give positive Benedict’s tests. Thus, Benedict’s test cannot be used to differentiate D-glucose from D-fructose as both glucose and fructose contain free carbonyl groups and will give positive tests. Hence, the option C ) is ruled out.
> Tollens reagent gives positive tests with aldehydes and alpha hydroxy ketones. Glucose being an aldehyde gives positive test with Tollen’s reagent. Fructose being alpha hydroxy ketone gives positive test with Tollen’s reagent. Hence, Tollen’s reagent cannot be used to distinguish glucose and fructose. Hence, the option B) is ruled out.
> Glucose gives a positive test with Fehling's solution. During the reaction with Fehling's solution, the base present converts fructose to glucose. Hence, fructose also gives a positive test with Fehling’s solution. Hence, glucose and fructose cannot be differentiated with Fehling’s solution. Hence, the option A ) is also ruled out.
> D-glucose and D-fructose can be differentiated by test with bromine water.
Bromine water is a mild oxidizing agent and can oxidize aldehydes to carboxylic acids. But bromine water cannot oxidize ketones to carboxylic acids.
Thus, bromine water can oxidise D-glucose to a carboxylic acid. But D-fructose will not be oxidised with bromine water.
Thus, bromine water can be used to distinguish between D-glucose and D-fructose.
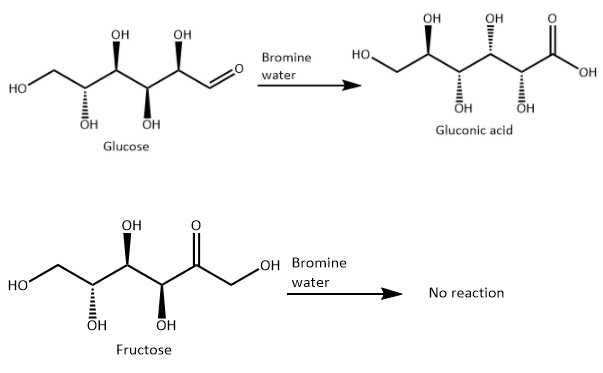
Hence, the option D ) is the correct option.
Note: D-glucose and D-fructose, both are simple carbohydrates They have one carbonyl group and five hydroxyl groups in their molecules. Both have same molecular formula of \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}\]. The only difference is that in glucose, the carbonyl group is the aldehyde group whereas in fructose, the carbonyl group is ketone.
Complete step by step answer:
> Select the reagent that can react with either glucose or fructose. The reagent should not react with both. Also during the selected reagent with base, inter-conversion of glucose and fructose should not happen.
> Benedict's test is used to tell if the sugar is reducing or not. When simple carbohydrates have free aldehyde or ketone functional groups, they give positive Benedict’s tests. Thus, Benedict’s test cannot be used to differentiate D-glucose from D-fructose as both glucose and fructose contain free carbonyl groups and will give positive tests. Hence, the option C ) is ruled out.
> Tollens reagent gives positive tests with aldehydes and alpha hydroxy ketones. Glucose being an aldehyde gives positive test with Tollen’s reagent. Fructose being alpha hydroxy ketone gives positive test with Tollen’s reagent. Hence, Tollen’s reagent cannot be used to distinguish glucose and fructose. Hence, the option B) is ruled out.
> Glucose gives a positive test with Fehling's solution. During the reaction with Fehling's solution, the base present converts fructose to glucose. Hence, fructose also gives a positive test with Fehling’s solution. Hence, glucose and fructose cannot be differentiated with Fehling’s solution. Hence, the option A ) is also ruled out.
> D-glucose and D-fructose can be differentiated by test with bromine water.
Bromine water is a mild oxidizing agent and can oxidize aldehydes to carboxylic acids. But bromine water cannot oxidize ketones to carboxylic acids.
Thus, bromine water can oxidise D-glucose to a carboxylic acid. But D-fructose will not be oxidised with bromine water.
Thus, bromine water can be used to distinguish between D-glucose and D-fructose.

Hence, the option D ) is the correct option.
Note: D-glucose and D-fructose, both are simple carbohydrates They have one carbonyl group and five hydroxyl groups in their molecules. Both have same molecular formula of \[{{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}\]. The only difference is that in glucose, the carbonyl group is the aldehyde group whereas in fructose, the carbonyl group is ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




