
Describe the Jahn Teller effect.
Answer
589.2k+ views
Hint: Jahn Teller effect is related to the distortion of a non linear molecular system. It explains the reduction in the symmetry and the energy of the system. Jahn Teller effect is due to the different extent of interactions of ligands with d orbitals of metals.
Complete answer:
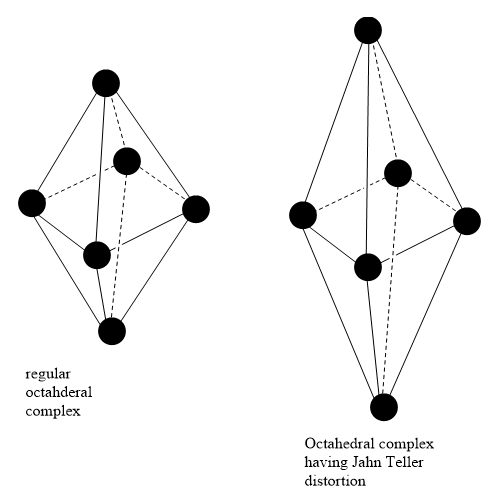
A non linear molecular system undergoes distortion. Due to this distortion, the symmetry and the energy of the system is reduced. This distortion is known as the Jahn Teller effect.
Octahedral complexes can have axial bond lengths different from equatorial bond lengths. Such complexes show the John Teller effect. Axial bond lengths can be greater than equatorial bond lengths. It is also possible that Axial bond lengths can be smaller than equatorial bond lengths. Tetrahedral complexes also show the Jahn Teller effect. This effect depends on the electronic state of the system.
Consider a nonlinear complex. If the electronic configuration in the ground state has degenerate orbitals, then, the complex will remove the degeneracy by distortion so as to achieve lower energy.
In the presence of an octahedral field, d orbitals of metal split into two levels, \[{t_{2g}}\] and \[{e_g}\]. \[{t_{2g}}\] has lower energy and is directed in between the axis so that it does not face the approaching ligands directly. \[{e_g}\] has higher energy and is directed along the axis so that it faces the approaching ligands directly.
The interaction between ligands and \[{e_g}\] orbitals is greater than that between ligands and \[{t_{2g}}\] orbitals, high spin \[{d^4}\], low spin \[{d^7}\] and \[{d^9}\] configurations have greater Jahn Teller effect.
Note: In high spin \[{d^4}\] octahedral complexes of \[{\text{C}}{{\text{r}}^{{\text{2 + }}}}{\text{ and C}}{{\text{u}}^{{\text{2 + }}}}\], Jahn Teller effect is observed as the lone pair of electrons split degenerate \[{e_g}\] level into \[{d_{{z^2}}}{\text{ }} and {\text{ }}{d_{{x^2} - {y^2}}}\] orbital.
Complete answer:
A non linear molecular system undergoes distortion. Due to this distortion, the symmetry and the energy of the system is reduced. This distortion is known as the Jahn Teller effect.
Octahedral complexes can have axial bond lengths different from equatorial bond lengths. Such complexes show the John Teller effect. Axial bond lengths can be greater than equatorial bond lengths. It is also possible that Axial bond lengths can be smaller than equatorial bond lengths. Tetrahedral complexes also show the Jahn Teller effect. This effect depends on the electronic state of the system.
Consider a nonlinear complex. If the electronic configuration in the ground state has degenerate orbitals, then, the complex will remove the degeneracy by distortion so as to achieve lower energy.
In the presence of an octahedral field, d orbitals of metal split into two levels, \[{t_{2g}}\] and \[{e_g}\]. \[{t_{2g}}\] has lower energy and is directed in between the axis so that it does not face the approaching ligands directly. \[{e_g}\] has higher energy and is directed along the axis so that it faces the approaching ligands directly.
The interaction between ligands and \[{e_g}\] orbitals is greater than that between ligands and \[{t_{2g}}\] orbitals, high spin \[{d^4}\], low spin \[{d^7}\] and \[{d^9}\] configurations have greater Jahn Teller effect.

Note: In high spin \[{d^4}\] octahedral complexes of \[{\text{C}}{{\text{r}}^{{\text{2 + }}}}{\text{ and C}}{{\text{u}}^{{\text{2 + }}}}\], Jahn Teller effect is observed as the lone pair of electrons split degenerate \[{e_g}\] level into \[{d_{{z^2}}}{\text{ }} and {\text{ }}{d_{{x^2} - {y^2}}}\] orbital.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




