
Cyclopentene on treatment with alkaline potassium permanganate gives
A. Cyclopentanol
B. Trans 1, 2-cyclopentanediol
C. Cis 1, 2-cyclopentanediol
D. 1:1 mixture of cis and Trans-1,2-cyclopentanediol
Answer
233.1k+ views
Hint: Cycloalkenes are ring compounds with one or more double bonds in the molecule. Cyclopentene is a type of cycloalkenes. Cyclopentene on treatment with alkaline potassium permanganate undergoes oxidation as alkaline potassium permanganate is a strong oxidising agent.
Complete Step by Step Answer:
Cycloalkenes are cyclic compounds possessing one or more double bonds in the molecule. Cyclopentene is a type of cycloalkenes.
Potassium manganate(VII) solution when added to sodium carbonate solution becomes little alkaline. The purple solution initially becomes dark green and then develops a dark brown precipitate. Alkaline potassium permanganate is a strong oxidising agent which undergoes reduction itself.
Under an alkaline climate, the manganate (VII) ions are initially reduced to green manganate (VI) ions.
Manganate(VII) ions are a powerful oxidising agent and oxidise cyclopentene to cis 1,2-cyclopentanediol.
The alkaline potassium manganate (VII) solution makes the cyclopentene undergo oxidation by breaking down the carbon-carbon double bond and altering it with two bonds in which carbon is attached to the hydroxyl group. This is a cis isomer because two bonds in which carbon is attached to the hydroxyl group
bonds are on the same side.
The reaction happens as follows:-
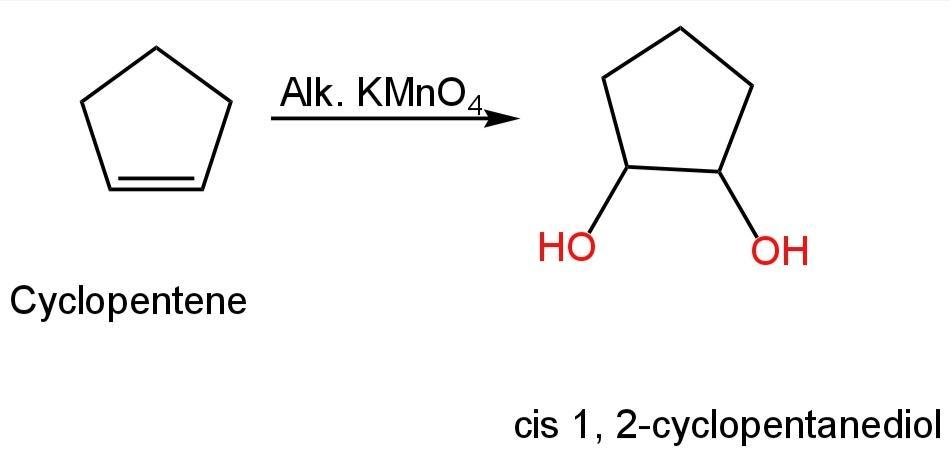
Image: Conversion of cyclopentene into cis 1, 2-cyclopentanediol.
So, option C is correct.
Note: Alkenes react with potassium manganate(VII) solution. It is a purple colour solution. The colour modification relies on whether the potassium manganate(VII) is utilised in acidic or alkaline conditions.If the potassium manganate(VII) solution is mixed with dilute sulfuric acid, the purple solution becomes colourless.
Complete Step by Step Answer:
Cycloalkenes are cyclic compounds possessing one or more double bonds in the molecule. Cyclopentene is a type of cycloalkenes.
Potassium manganate(VII) solution when added to sodium carbonate solution becomes little alkaline. The purple solution initially becomes dark green and then develops a dark brown precipitate. Alkaline potassium permanganate is a strong oxidising agent which undergoes reduction itself.
Under an alkaline climate, the manganate (VII) ions are initially reduced to green manganate (VI) ions.
Manganate(VII) ions are a powerful oxidising agent and oxidise cyclopentene to cis 1,2-cyclopentanediol.
The alkaline potassium manganate (VII) solution makes the cyclopentene undergo oxidation by breaking down the carbon-carbon double bond and altering it with two bonds in which carbon is attached to the hydroxyl group. This is a cis isomer because two bonds in which carbon is attached to the hydroxyl group
bonds are on the same side.
The reaction happens as follows:-

Image: Conversion of cyclopentene into cis 1, 2-cyclopentanediol.
So, option C is correct.
Note: Alkenes react with potassium manganate(VII) solution. It is a purple colour solution. The colour modification relies on whether the potassium manganate(VII) is utilised in acidic or alkaline conditions.If the potassium manganate(VII) solution is mixed with dilute sulfuric acid, the purple solution becomes colourless.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




