
How will you convert benzaldehyde into following compounds? More than one step be required.
(i) Benzophenone
(ii) Benzoic acid
Answer
582.6k+ views
Hint: Benzaldehyde is a compound having an aldehyde group attached to benzene. Benzophenone is a compound having two benzene groups connected with a carbonyl group. While benzoic acid is a compound having carboxyl groups attached to benzene. Benzene to benzoic acid is a simple step conversion while the other involves two to three steps.
Complete step by step answer:
(i) When aldehyde is oxidized, a carboxylic acid is obtained. Benzaldehyde is the simplest aromatic aldehyde. It has an almond-like odor. Benzoic acid is a colorless solid. The chemical formula of benzoic acid is ${{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}$.
Thus benzaldehyde is converted to benzophenone in three steps:
Oxidation of benzaldehyde to benzoic acid.
Benzaldehyde is oxidized to benzoic acid in the presence of alkaline potassium permanganate. Since benzaldehyde possesses a benzene ring, it facilitates the electrophilic substitution reaction. The chemical equation for oxidation of benzaldehyde to benzoic acid is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{CHO}}\xrightarrow{{{\text{KMn}}{{\text{O}}_4}}}{{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}$
Benzaldehyde Benzoic acid
Conversion of benzoic acid to benzene.
Benzoic acid is heated with soda lime which is a mixture of ${\text{NaOH}}$ and ${\text{CaO}}$, benzene is obtained. The reaction is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}\xrightarrow[{{\text{CaO}}}]{{{\text{NaOH}}}}{{\text{C}}_6}{{\text{H}}_6}$
Conversion of benzene to benzophenone.
Benzene is reacted with benzoyl chloride, ${{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}$ in the presence of ${\text{Al}}{{\text{Cl}}_3}$, benzophenone, ${{\text{C}}_6}{{\text{H}}_5}{\text{CO}}{{\text{C}}_6}{{\text{H}}_5}$ is obtained. The chemical equation is given below:
${{\text{C}}_6}{{\text{H}}_6} + {{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}\xrightarrow{{{\text{Al}}{{\text{Cl}}_3}}}{{\text{C}}_6}{{\text{H}}_5}{\text{CO}}{{\text{C}}_6}{{\text{H}}_5}$
This reaction is called Friedel Crafts acylation.
Note:
Benzaldehyde can also be converted to benzoic acid by Cannizaro reaction. Cannizaro reaction is a type of disproportionation reaction. This involves disproportionation of an aldehyde which lacks $\alpha - $ hydrogen atom to salt of an acid and a primary alcohol. The reaction is given below:
$2$
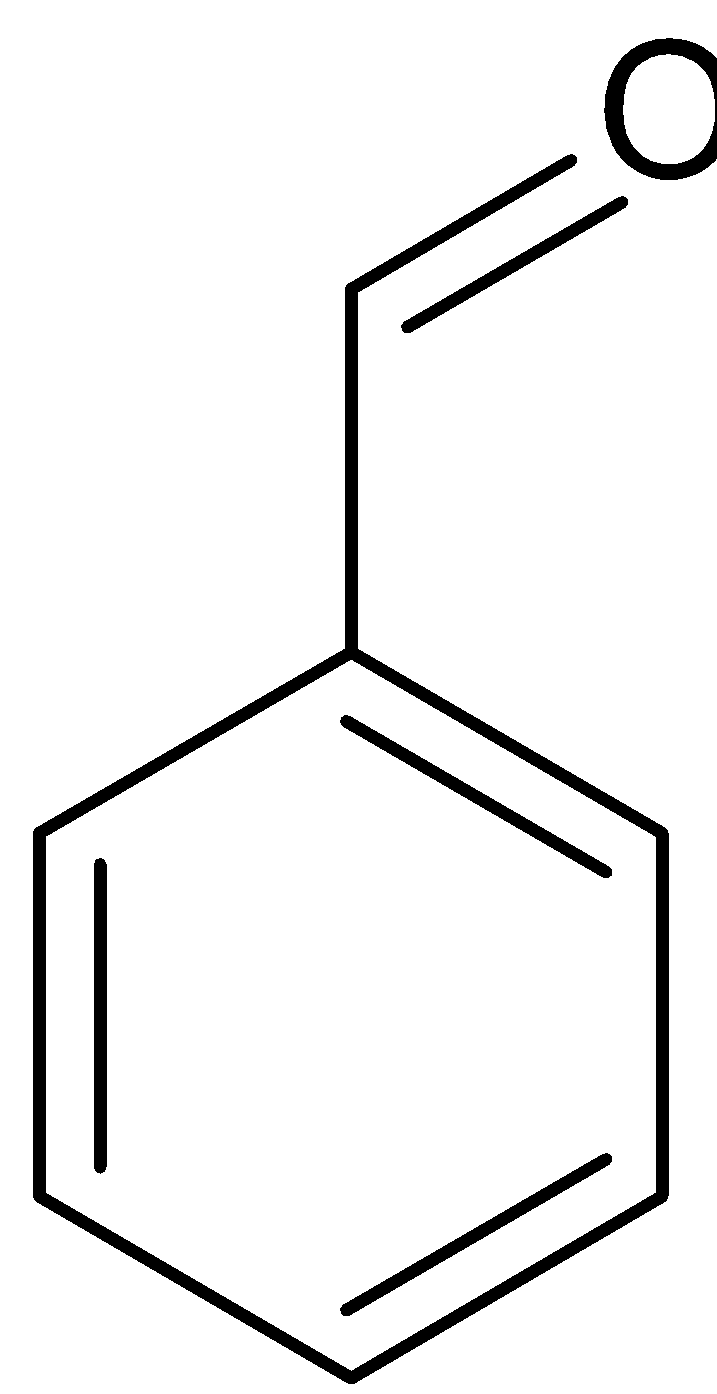 $ + {\text{NaOH}} \to $
$ + {\text{NaOH}} \to $
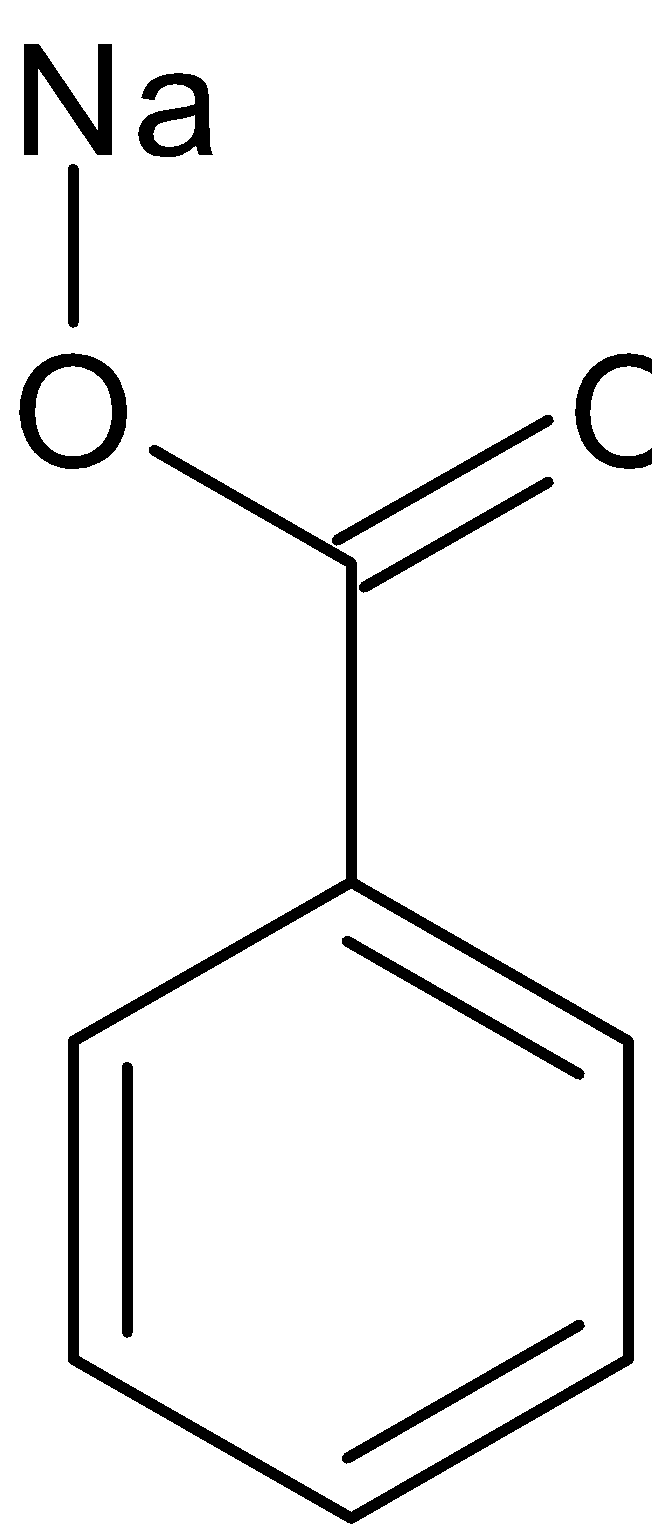
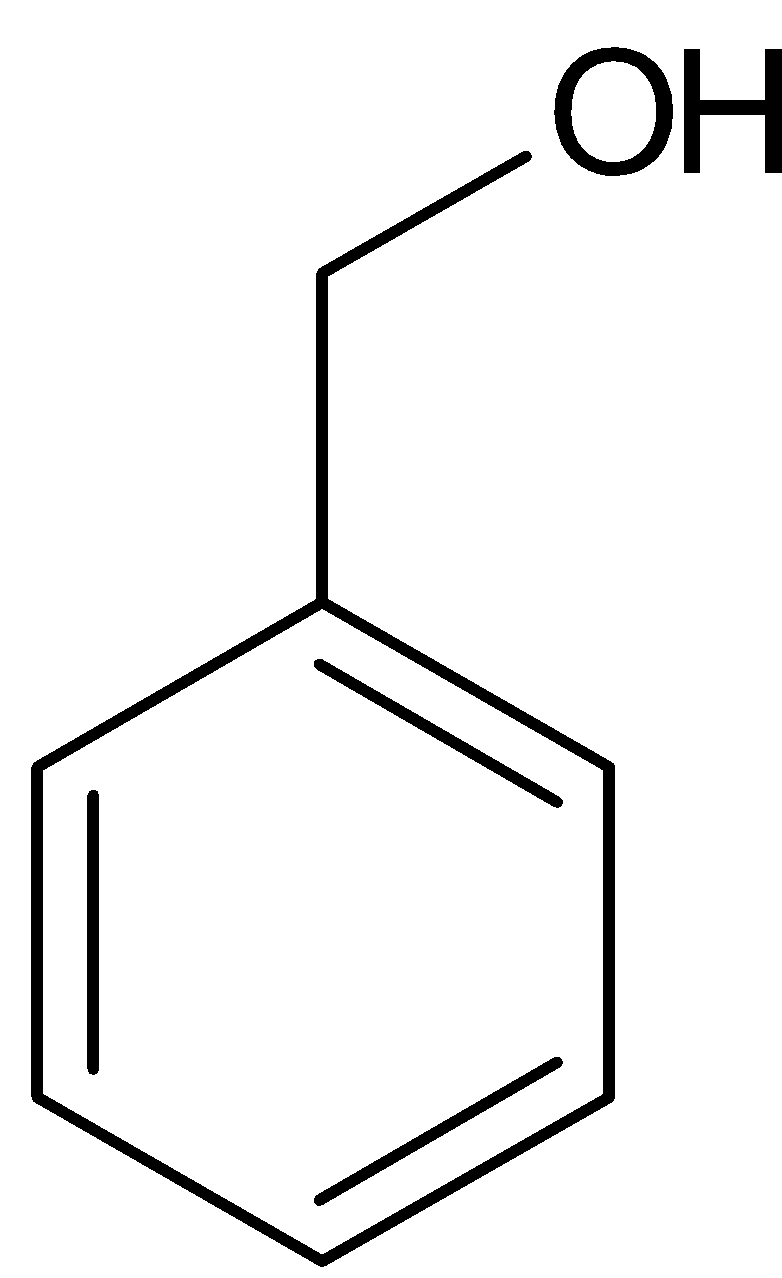 $ + $
$ + $
 $\xrightarrow{{{{\text{H}}^ + }}}$
$\xrightarrow{{{{\text{H}}^ + }}}$
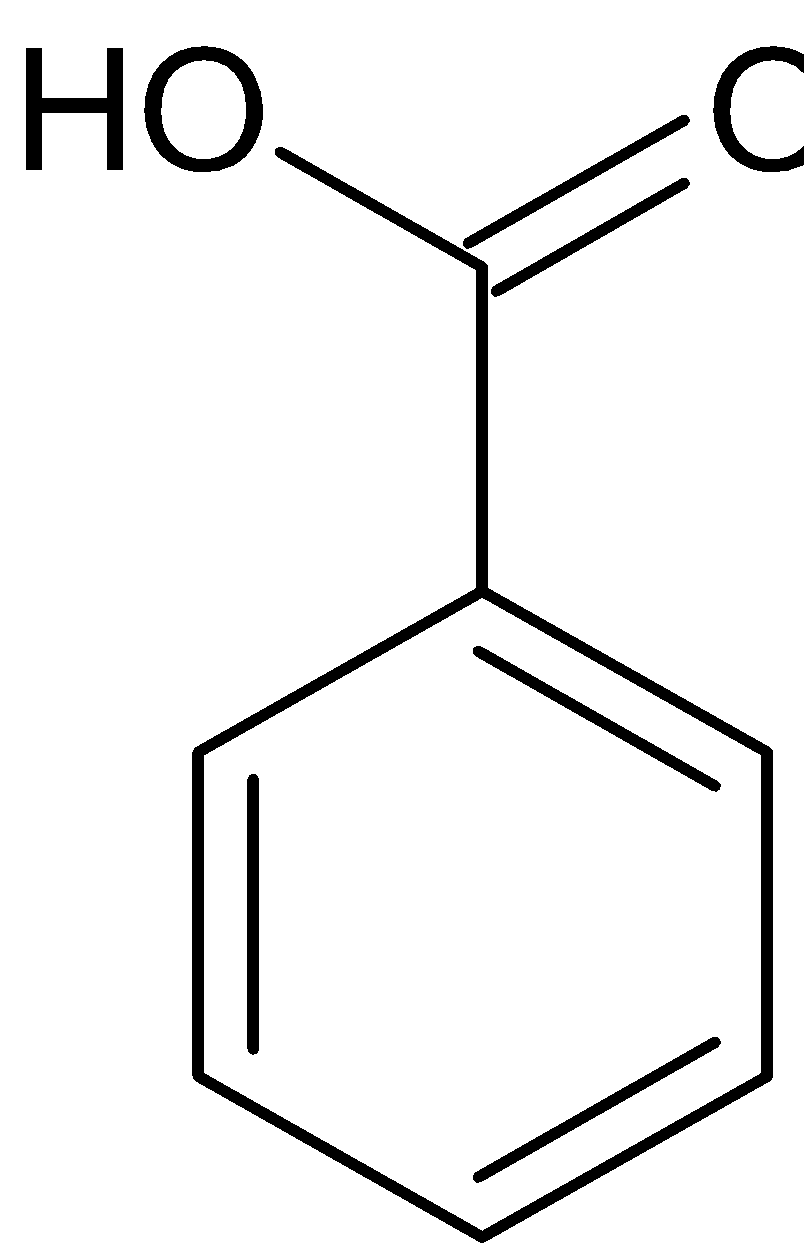
Benzaldehyde Benzyl alcohol Sodium benzoate
Complete step by step answer:
(i) When aldehyde is oxidized, a carboxylic acid is obtained. Benzaldehyde is the simplest aromatic aldehyde. It has an almond-like odor. Benzoic acid is a colorless solid. The chemical formula of benzoic acid is ${{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}$.
Thus benzaldehyde is converted to benzophenone in three steps:
Oxidation of benzaldehyde to benzoic acid.
Benzaldehyde is oxidized to benzoic acid in the presence of alkaline potassium permanganate. Since benzaldehyde possesses a benzene ring, it facilitates the electrophilic substitution reaction. The chemical equation for oxidation of benzaldehyde to benzoic acid is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{CHO}}\xrightarrow{{{\text{KMn}}{{\text{O}}_4}}}{{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}$
Benzaldehyde Benzoic acid
Conversion of benzoic acid to benzene.
Benzoic acid is heated with soda lime which is a mixture of ${\text{NaOH}}$ and ${\text{CaO}}$, benzene is obtained. The reaction is given below:
${{\text{C}}_6}{{\text{H}}_5}{\text{COOH}}\xrightarrow[{{\text{CaO}}}]{{{\text{NaOH}}}}{{\text{C}}_6}{{\text{H}}_6}$
Conversion of benzene to benzophenone.
Benzene is reacted with benzoyl chloride, ${{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}$ in the presence of ${\text{Al}}{{\text{Cl}}_3}$, benzophenone, ${{\text{C}}_6}{{\text{H}}_5}{\text{CO}}{{\text{C}}_6}{{\text{H}}_5}$ is obtained. The chemical equation is given below:
${{\text{C}}_6}{{\text{H}}_6} + {{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}\xrightarrow{{{\text{Al}}{{\text{Cl}}_3}}}{{\text{C}}_6}{{\text{H}}_5}{\text{CO}}{{\text{C}}_6}{{\text{H}}_5}$
This reaction is called Friedel Crafts acylation.
Note:
Benzaldehyde can also be converted to benzoic acid by Cannizaro reaction. Cannizaro reaction is a type of disproportionation reaction. This involves disproportionation of an aldehyde which lacks $\alpha - $ hydrogen atom to salt of an acid and a primary alcohol. The reaction is given below:
$2$




Benzaldehyde Benzyl alcohol Sodium benzoate
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




