
Convert :
1.) Phenyl Cyanide from Benzene
2.) Phenol from Toluene
Answer
573.9k+ views
Hint: There are many paths by which benzene can be converted into phenyl cyanide. It may involve first substitution of methyl group on benzene ring and then its oxidation and then further conversion with use of reagents. Similarly, toluene can be oxidised first and then converted into phenol.
Complete Solution :
There are many methods of formation of phenyl cyanide from benzene.
Benzene is an electron rich compound. It is resonance stabilised , so it can not undergo addition reactions because addition will lead to destroy of resonance. But it undergoes electrophilic substitution reactions where one Hydrogen is substituted by any other group.
The reaction for benzene to phenyl cyanide is as-
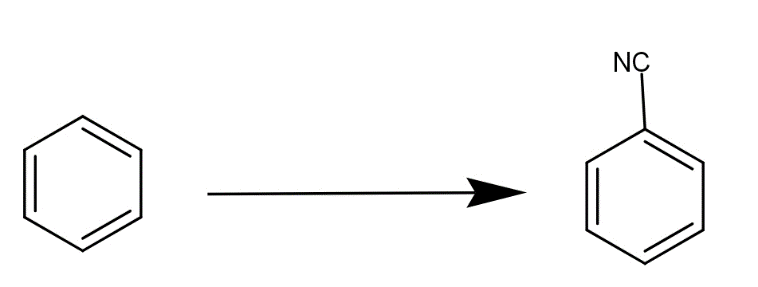
But it requires a series of steps to give this product.
The steps involved are -
The first step involves the Friedel Crafts alkylation reaction in which methyl chloride and $AlC{l_3}$ are used to convert benzene into toluene.
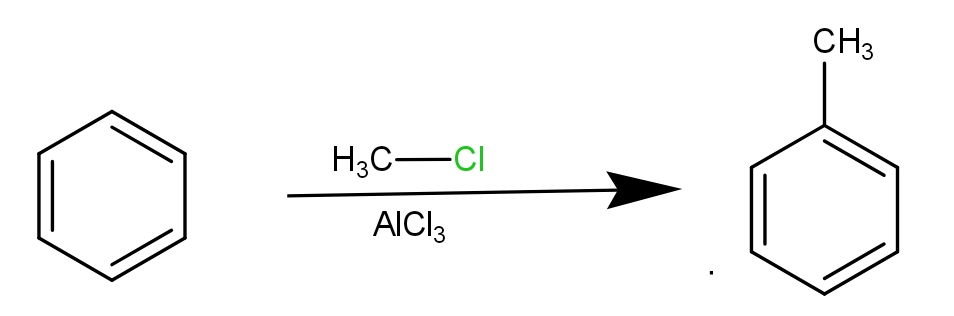
Then, the Toluene is converted to benzoic acid by acidifying Potassium dichromate. The reaction is as -
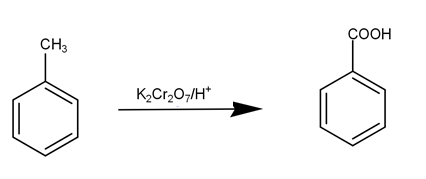
This benzoic acid is then converted into benzamide by reaction of benzoic acid with ammonia as-
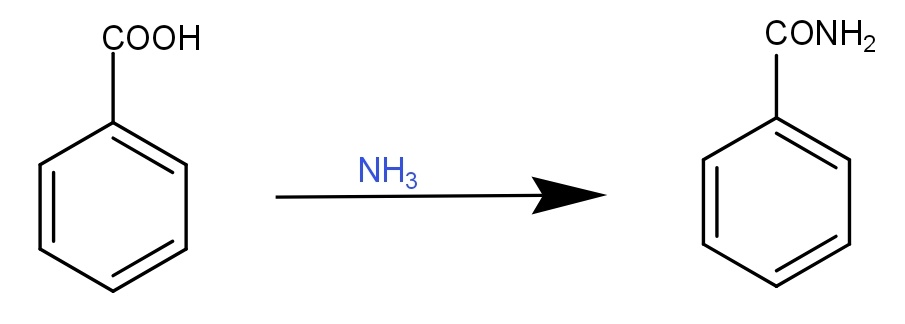
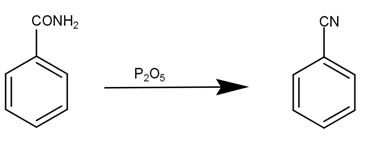
Then, this benzamide is reduced by phosphorus pentoxide to give phenyl cyanide, our desired product. The reaction can be given as -
The second reaction is conversion of toluene to phenol. This reaction can be written as -

- It also involves two steps that are as-
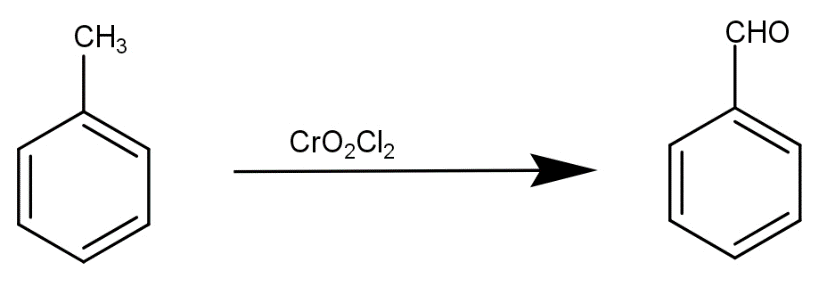
The first step is conversion of toluene to benzaldehyde by reaction with $Cr{O_2}C{l_2}$. This reaction is written as-
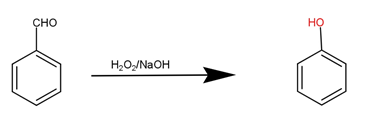
The second step is the reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide to produce phenol that is our desired product.
Note: The conversion of toluene to benzaldehyde by use of reagent $Cr{O_2}C{l_2}$ is termed as Etard reaction. This reaction is used to produce benzaldehyde for many purposes. The reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide is named as Dakin reaction. It is commonly used to prepare phenol.
Complete Solution :
There are many methods of formation of phenyl cyanide from benzene.
Benzene is an electron rich compound. It is resonance stabilised , so it can not undergo addition reactions because addition will lead to destroy of resonance. But it undergoes electrophilic substitution reactions where one Hydrogen is substituted by any other group.
The reaction for benzene to phenyl cyanide is as-

But it requires a series of steps to give this product.
The steps involved are -
The first step involves the Friedel Crafts alkylation reaction in which methyl chloride and $AlC{l_3}$ are used to convert benzene into toluene.

Then, the Toluene is converted to benzoic acid by acidifying Potassium dichromate. The reaction is as -

This benzoic acid is then converted into benzamide by reaction of benzoic acid with ammonia as-

Then, this benzamide is reduced by phosphorus pentoxide to give phenyl cyanide, our desired product. The reaction can be given as -

The second reaction is conversion of toluene to phenol. This reaction can be written as -

- It also involves two steps that are as-
The first step is conversion of toluene to benzaldehyde by reaction with $Cr{O_2}C{l_2}$. This reaction is written as-

The second step is the reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide to produce phenol that is our desired product.

Note: The conversion of toluene to benzaldehyde by use of reagent $Cr{O_2}C{l_2}$ is termed as Etard reaction. This reaction is used to produce benzaldehyde for many purposes. The reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide is named as Dakin reaction. It is commonly used to prepare phenol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




