
Consider the direction of the current And flow of electrons from the section A to B that goes through the resistor in the circuit pictured.
A. Current flow: Upward
Electron flow: Downward
B. Current flow: Upward
Electron flow: Upward
C. Current flow: Downward
Electron flow: Downward
D. Current flow: Downward
Electron flow: Upward

Answer
553.8k+ views
Hint: Typical cells are made of chemical solution called electrolyte and two electrodes where one will be positive electrode and the other will be negative electrode. a combination of cells is called a battery. Chemical reaction occurs between them to generate the electromotive force. Flow of electrons happens between them.
Complete step by step answer:
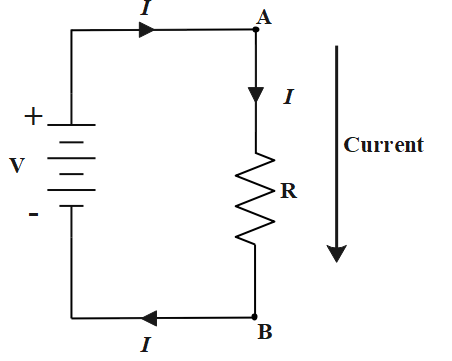
Inside the battery electrons travel from positive terminal to negative terminal whereas outside the battery electrons travel from negative terminal to positive terminal through a load resistor. Since electrons are of negative charge direction of flow of electrons is not considered as the flow of current.
Direction opposite to the flow of electrons in an external circuit i.e circuit which contains a load resistor is considered as the flow of conventional current.
If we consider points A and B, A is connected to the positive terminal whereas B is connected to the negative terminal. So electrons will be flowing from B to A whereas current will be flowing from A to B.
Hence electron flow will be in upward direction whereas current flow will be in downward direction.
Hence answer would be option D.
Additional information:
Two electrodes which are present in the cell are called cathode and anode. During discharging negative terminal is called anode while positive terminal is called cathode. Oxidation is loose of electrons while reduction means gain of electrons. In case of discharging oxidation occurs at negative terminal which behaves as anode while in case of charging reduction occurs at positive terminal which behaves as cathode.
Note: When battery is connected to bulb through a switch and if switch is turned on it is not like one electron is reaching from anode to cathode in external circuit. It is like one electron gets energized and causes vibration on adjacent electrons and this process continues hence the bulb gets on very fast. It is like a dominos effect and can be compared to the heat conduction process.
Complete step by step answer:

Inside the battery electrons travel from positive terminal to negative terminal whereas outside the battery electrons travel from negative terminal to positive terminal through a load resistor. Since electrons are of negative charge direction of flow of electrons is not considered as the flow of current.
Direction opposite to the flow of electrons in an external circuit i.e circuit which contains a load resistor is considered as the flow of conventional current.
If we consider points A and B, A is connected to the positive terminal whereas B is connected to the negative terminal. So electrons will be flowing from B to A whereas current will be flowing from A to B.
Hence electron flow will be in upward direction whereas current flow will be in downward direction.
Hence answer would be option D.
Additional information:
Two electrodes which are present in the cell are called cathode and anode. During discharging negative terminal is called anode while positive terminal is called cathode. Oxidation is loose of electrons while reduction means gain of electrons. In case of discharging oxidation occurs at negative terminal which behaves as anode while in case of charging reduction occurs at positive terminal which behaves as cathode.
Note: When battery is connected to bulb through a switch and if switch is turned on it is not like one electron is reaching from anode to cathode in external circuit. It is like one electron gets energized and causes vibration on adjacent electrons and this process continues hence the bulb gets on very fast. It is like a dominos effect and can be compared to the heat conduction process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




