
When compound X is heated with ${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}{\text{/}}{{\text{H}}^{\text{ + }}}$, a color change from orange to green is observed. Two tests are carried out on the organic product of this reaction:
Test Result Tollens test No change ${\text{2,4 - dinitrophenylhydrazine}}$ Orange precipitate
What is the compound?
(A)
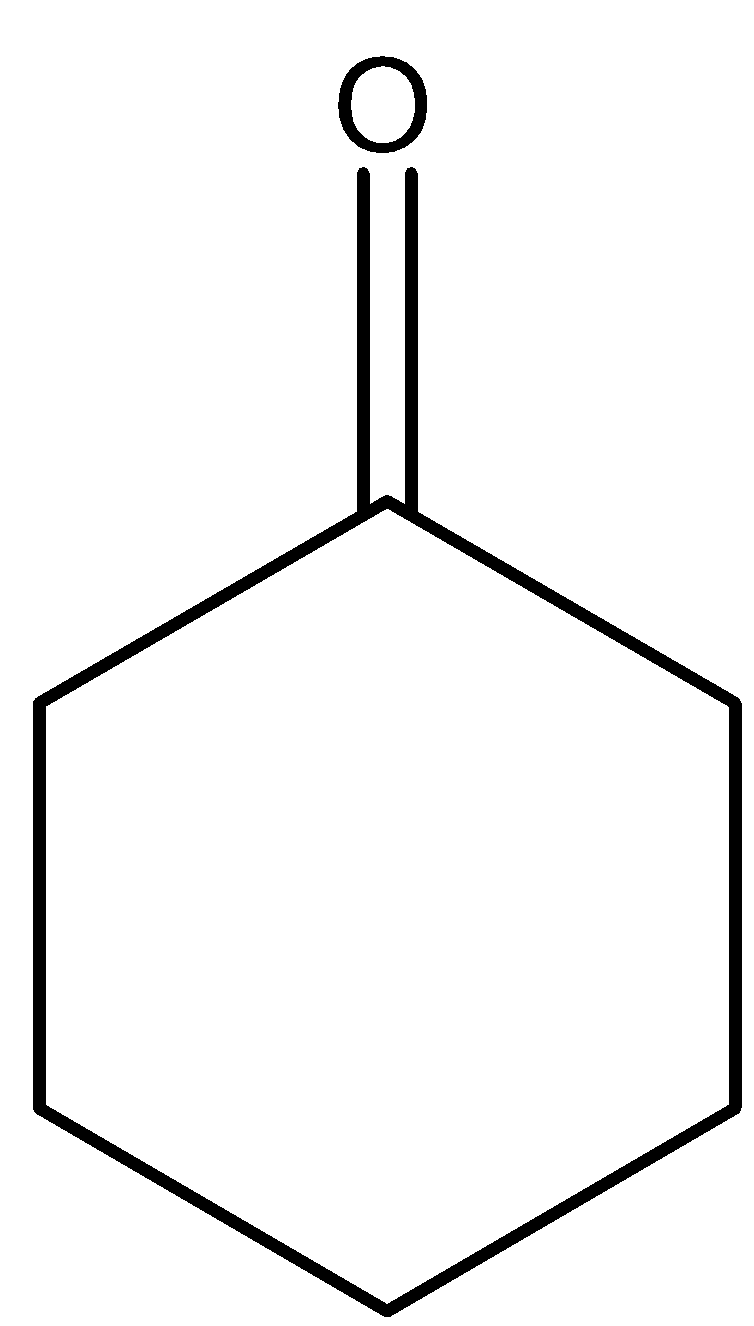
(B)
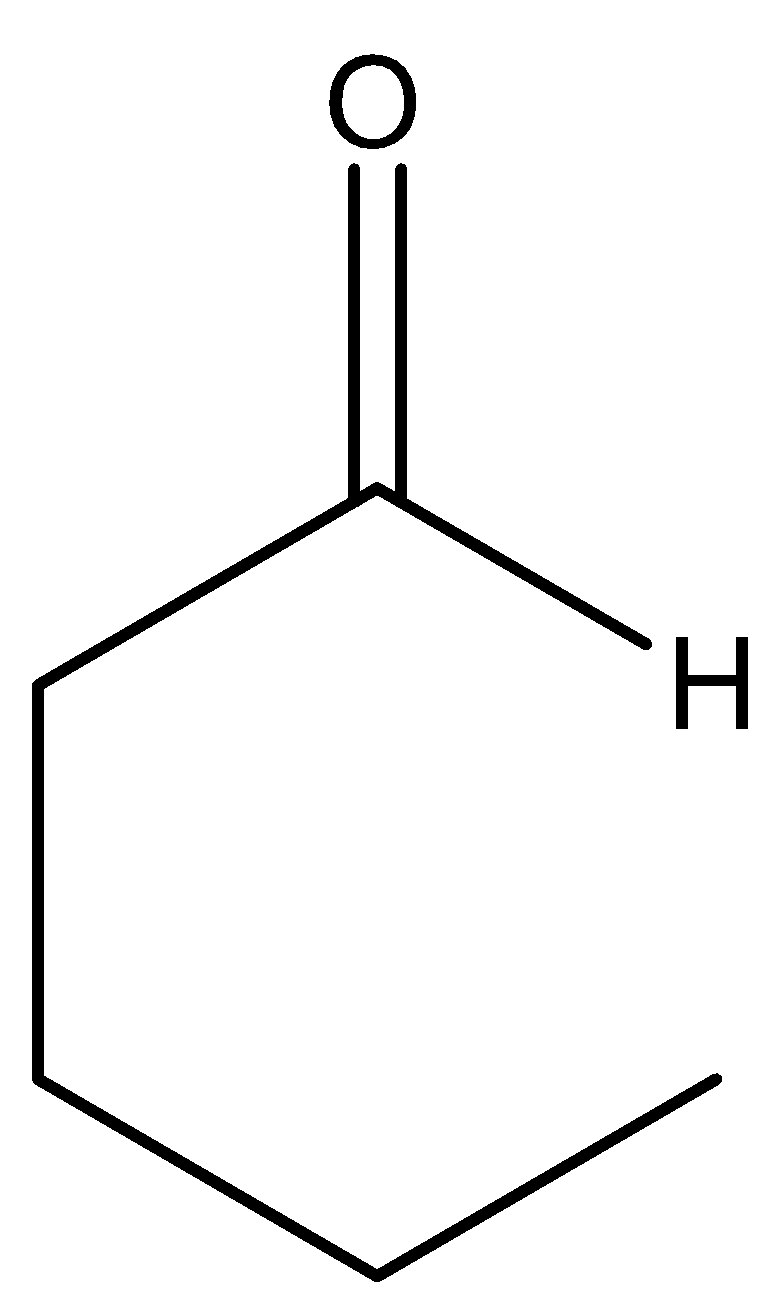
(C)
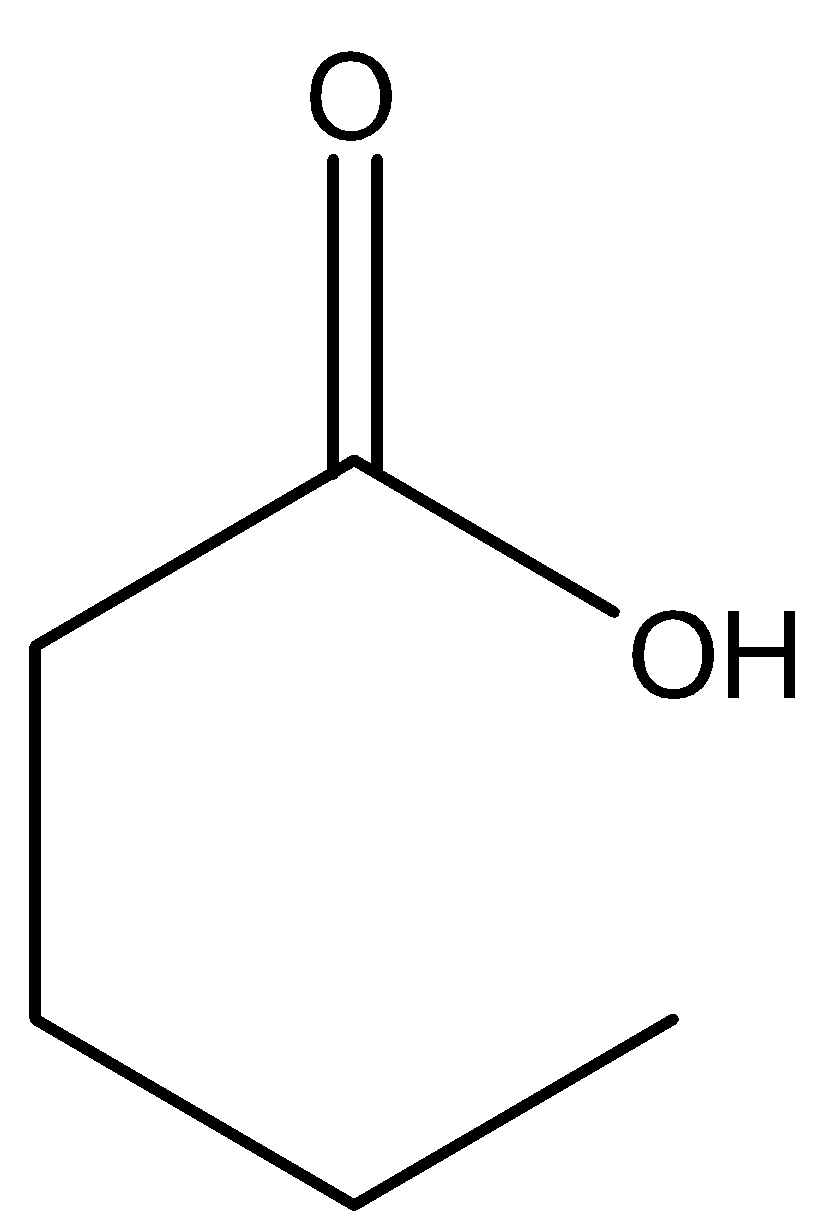
(D)
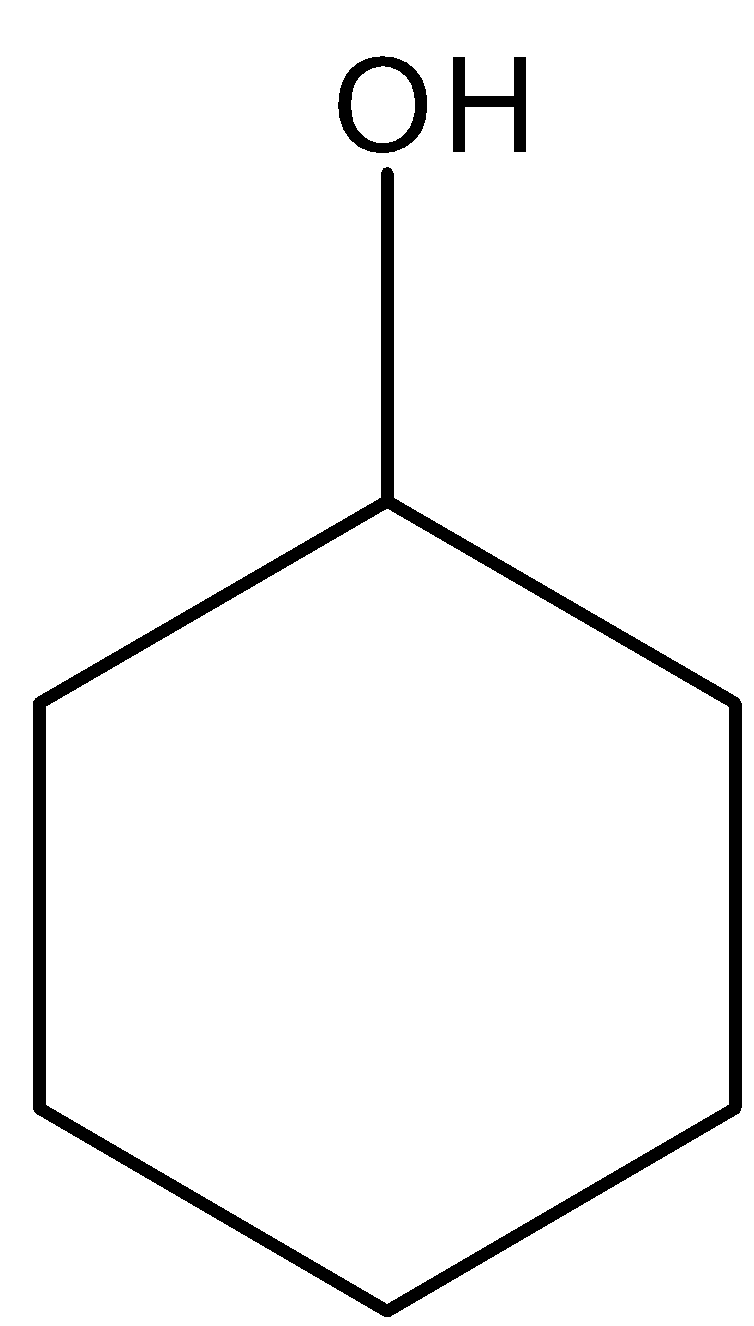
| Test | Result |
| Tollens test | No change |
| ${\text{2,4 - dinitrophenylhydrazine}}$ | Orange precipitate |




Answer
565.2k+ views
Hint: First we will see what is the product form after the reaction. Dichromate anion in acidic medium does oxidation of the substrate it acts upon. We will predict the product with the help of the functional group test given by it.
Complete step by step answer:
The dichromate anion is used to oxidize a substrate. It is a powerful oxidizing agent.
Dichromate oxidizes aldehyde into carboxylic acid.
It oxidizes primary alcohol to aldehyde and further into carboxylic acid.
It oxidizes secondary alcohol to ketones.
It does not oxidize ketones.
So the reactant cannot be a ketone as the reaction proceeds. The reaction of dichromate with aldehyde or alcohol changes color from orange to green. So, the reactant must be an aldehyde or alcohol.
TOLLENS TEST: It is a test for identification of aldehyde and ${\alpha - hydroxyketone}$. Since the product doesn't give a positive tollens test that means the product is not an aldehyde.
${\text{2,4 - dinitrophenylhydrazine}}$ is a test for ketone and aldehyde functional groups. The solution turns to orange when ketone or aldehyde is present.
So the product gives negative tollens test and positive DNP test it confirms product is ketone
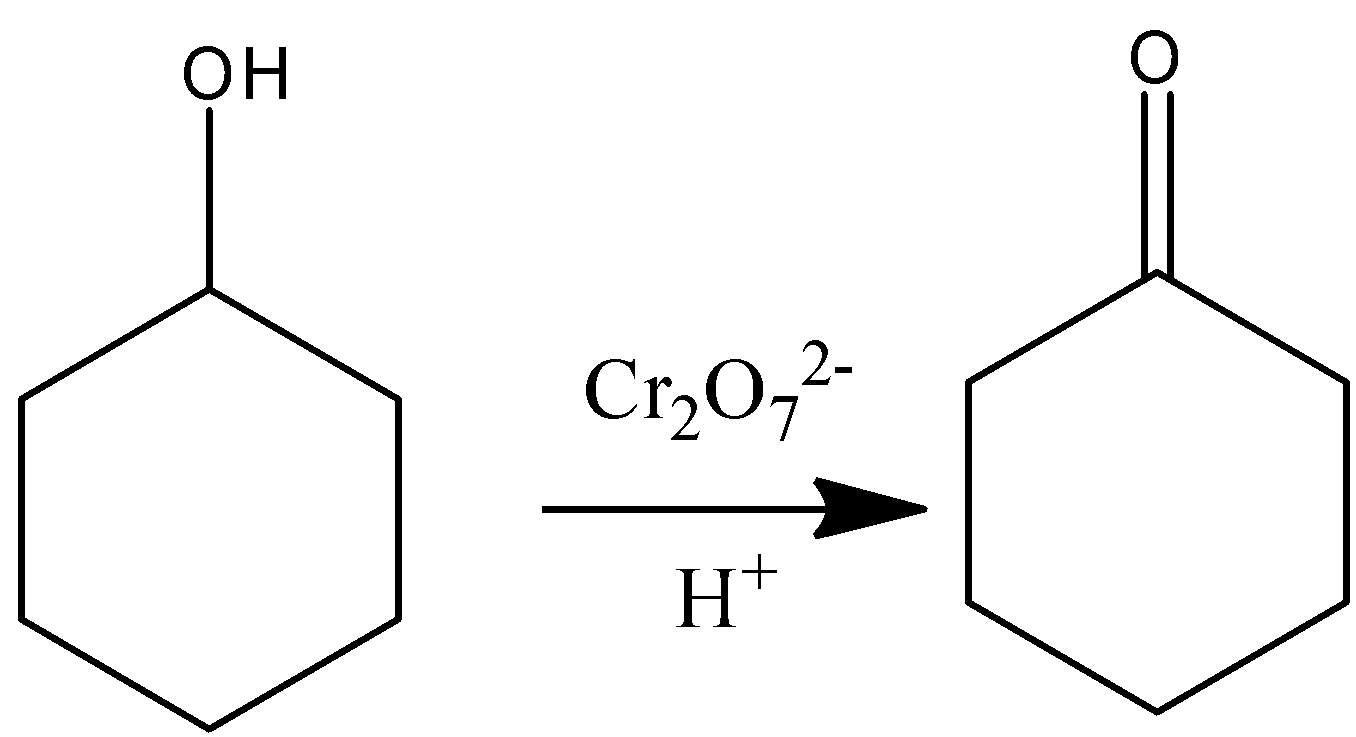
So, the correct answer is Option A.
Additional information:
The Dinitrophenyl hydrazine reacts with ketones and aldehyde only. It gives red, yellow or orange precipitate due to formation of phenyl hydrazone. So this test confirms that the substrate is either aldehyde or ketone.
Note: Potassium dichromate is a very strong oxidizing agent because in this molecule the Chromium cation is present in ${\text{ + 6}}$ oxidation state which makes it highly electron loving molecule which readily accepts electrons of other and oxidizes them.
Complete step by step answer:
The dichromate anion is used to oxidize a substrate. It is a powerful oxidizing agent.
Dichromate oxidizes aldehyde into carboxylic acid.
It oxidizes primary alcohol to aldehyde and further into carboxylic acid.
It oxidizes secondary alcohol to ketones.
It does not oxidize ketones.
So the reactant cannot be a ketone as the reaction proceeds. The reaction of dichromate with aldehyde or alcohol changes color from orange to green. So, the reactant must be an aldehyde or alcohol.
TOLLENS TEST: It is a test for identification of aldehyde and ${\alpha - hydroxyketone}$. Since the product doesn't give a positive tollens test that means the product is not an aldehyde.
${\text{2,4 - dinitrophenylhydrazine}}$ is a test for ketone and aldehyde functional groups. The solution turns to orange when ketone or aldehyde is present.
So the product gives negative tollens test and positive DNP test it confirms product is ketone

So, the correct answer is Option A.
Additional information:
The Dinitrophenyl hydrazine reacts with ketones and aldehyde only. It gives red, yellow or orange precipitate due to formation of phenyl hydrazone. So this test confirms that the substrate is either aldehyde or ketone.
Note: Potassium dichromate is a very strong oxidizing agent because in this molecule the Chromium cation is present in ${\text{ + 6}}$ oxidation state which makes it highly electron loving molecule which readily accepts electrons of other and oxidizes them.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




