
Compound ‘R’ on heating with \[{\text{aq}}{\text{.NaOH}}\] evolves \[{\text{N}}{{\text{H}}_{\text{3}}}\] gas.On heating with bromine in\[{\text{aq}}{\text{.NaOH}}\] evolves \[{\text{C}}{{\text{O}}_{\text{2}}}\] gas.With \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{/HCl}}\],it evolves \[{{\text{N}}_{\text{2}}}\] gas.Compound ‘R’ is;
A.
B.
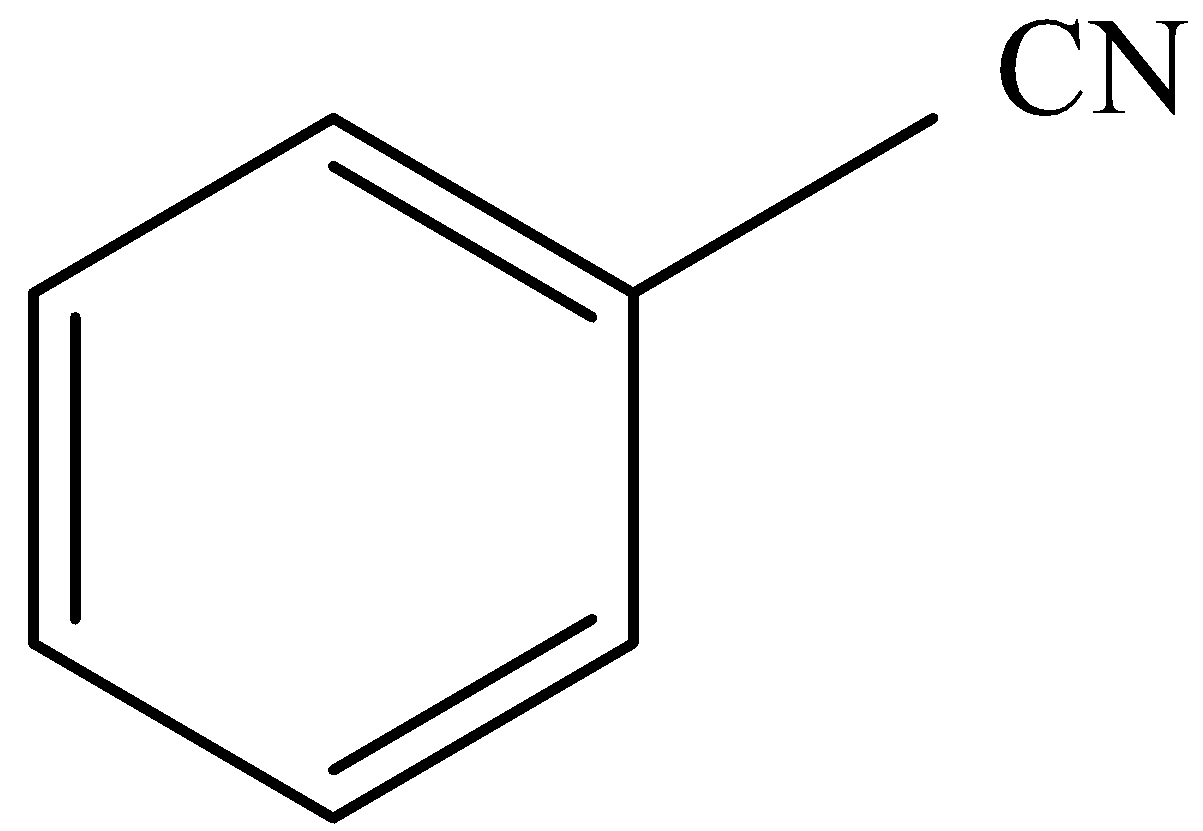
.
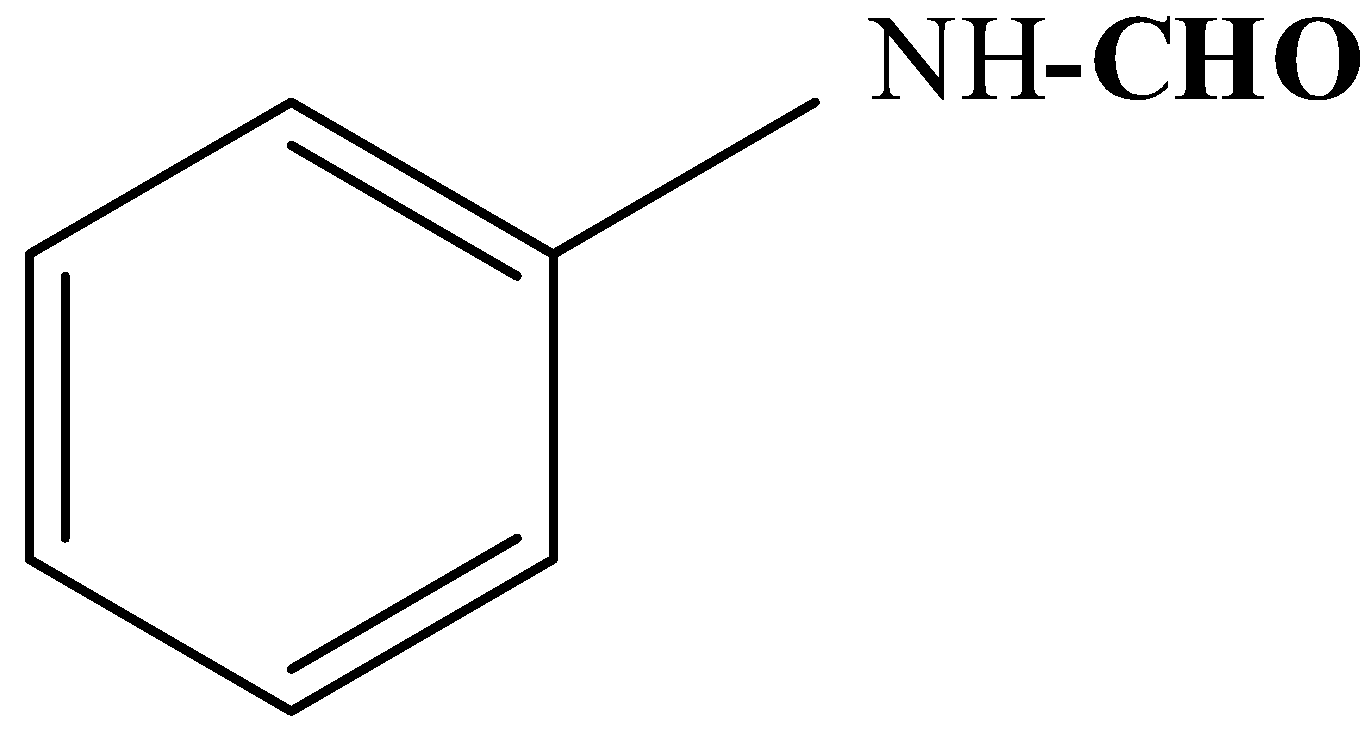
C.
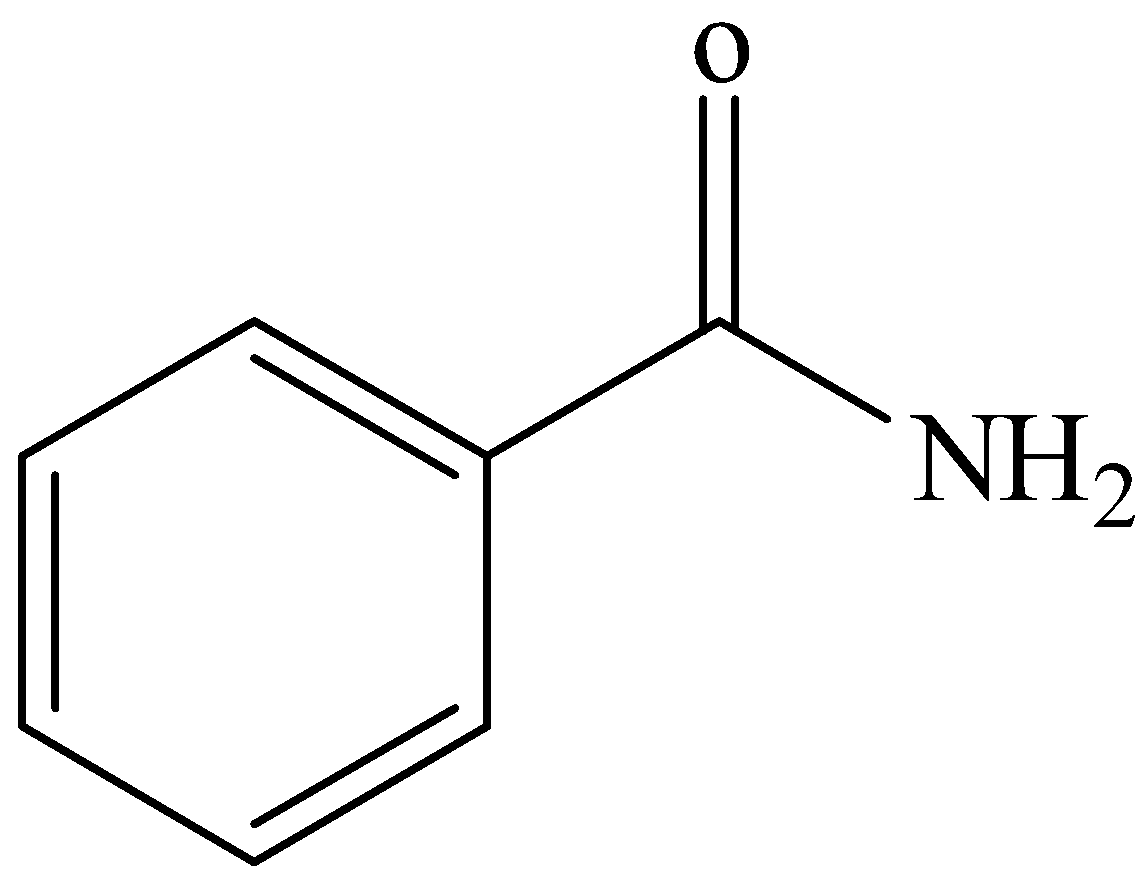
D.
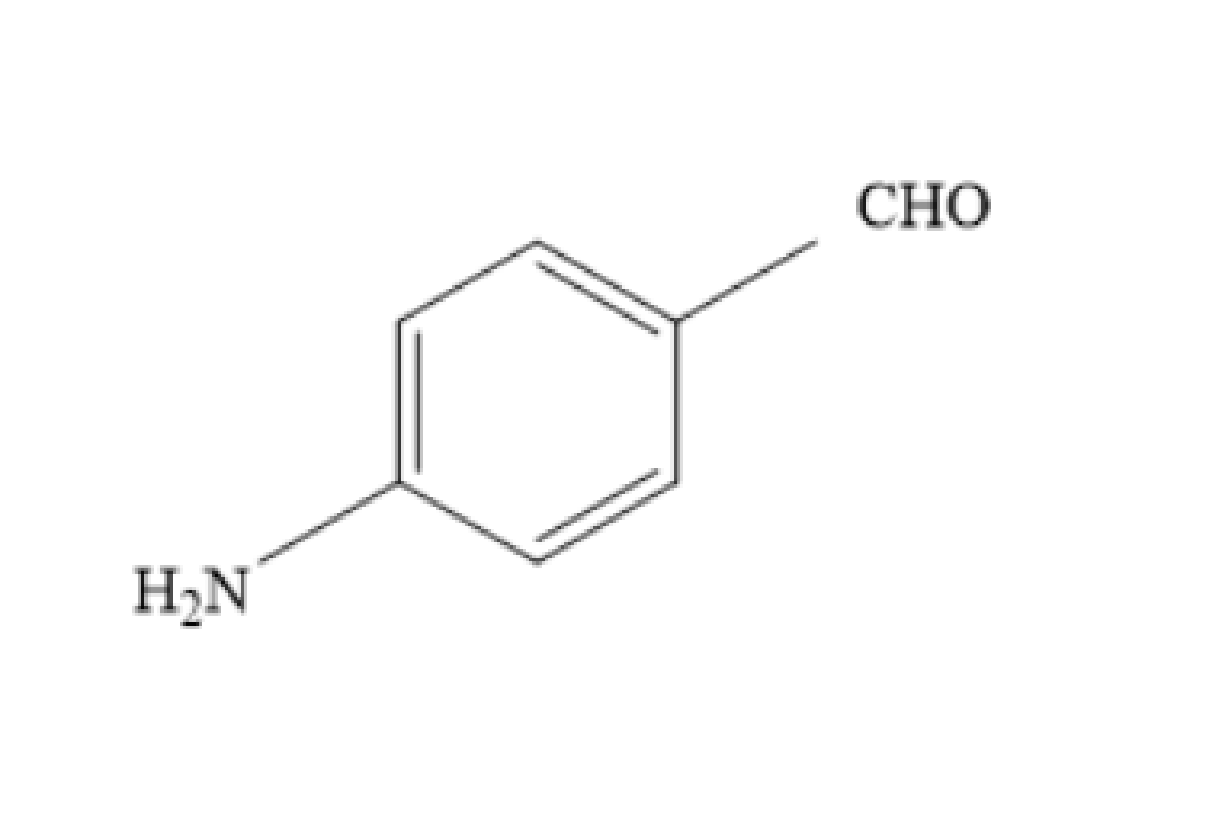




Answer
573.6k+ views
Hint: The above stated compound R will be an aromatic compound. It undergoes mainly three reactions. At first it is hydrolysed when heated with \[{\text{aq}}{\text{.NaOH}}\] . Then it undergoes Hoffmann Bromamide reaction. Finally, it is diazotized when treated with \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{/HCl}}\]. The compound that undergoes these reactions will be surely an aromatic compound.
Complete step by step answer:
The compound when heated with \[{\text{aq}}{\text{.NaOH}}\] gives the \[{\text{N}}{{\text{H}}_{\text{3}}}\] gas which means that there is the presence of an -\[{\text{N}}{{\text{H}}_{\text{2}}}\] group.
The reaction with heating the compound with Bromine in \[{\text{aq}}{\text{.NaOH}}\] is the Hoffmann Bromamide reaction. In this reaction the amides are converted to primary amines with one carbon atom less than the parent amide. Here \[{\text{RCON}}{{\text{H}}_{\text{2}}}\] gets converted to \[{\text{RN}}{{\text{H}}_{\text{2}}}\].
And finally, when this \[{\text{RN}}{{\text{H}}_{\text{2}}}\] is treated with \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{/HCl}}\], the diazonium salt of the corresponding compound is formed.
From these reactions we can make sure that the compound contains a- \[{\text{CON}}{{\text{H}}_{\text{2}}}\] group and it is an aromatic compound. Therefore, the compound R will be benzamide. The three reactions can be shown as below:
1.Heating with \[{\text{aq}}{\text{.NaOH}}\]
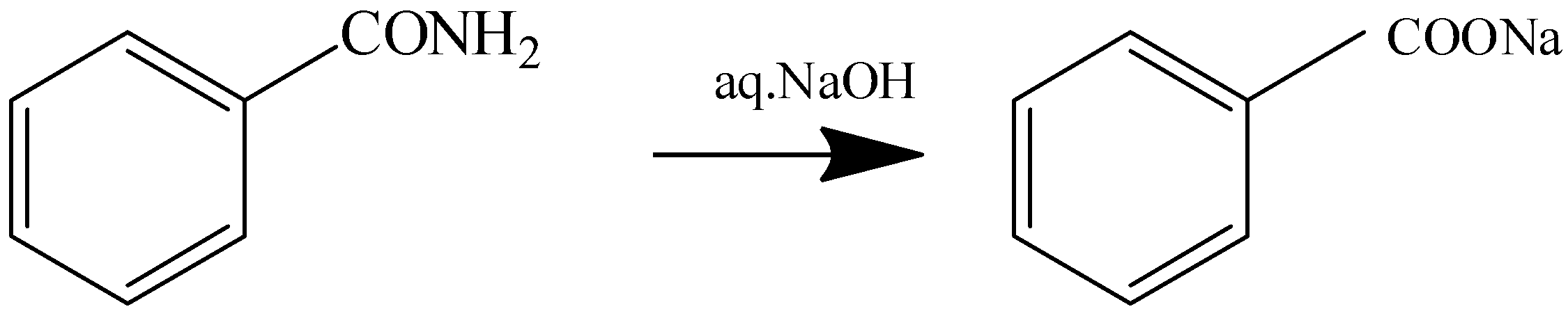
2.Hoffmann Bromamide Reaction
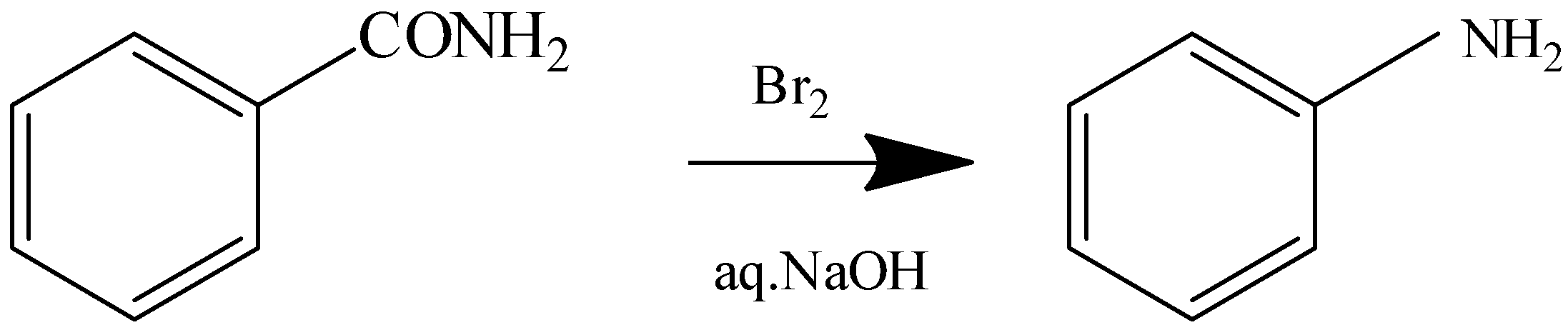
3.Diazotisation
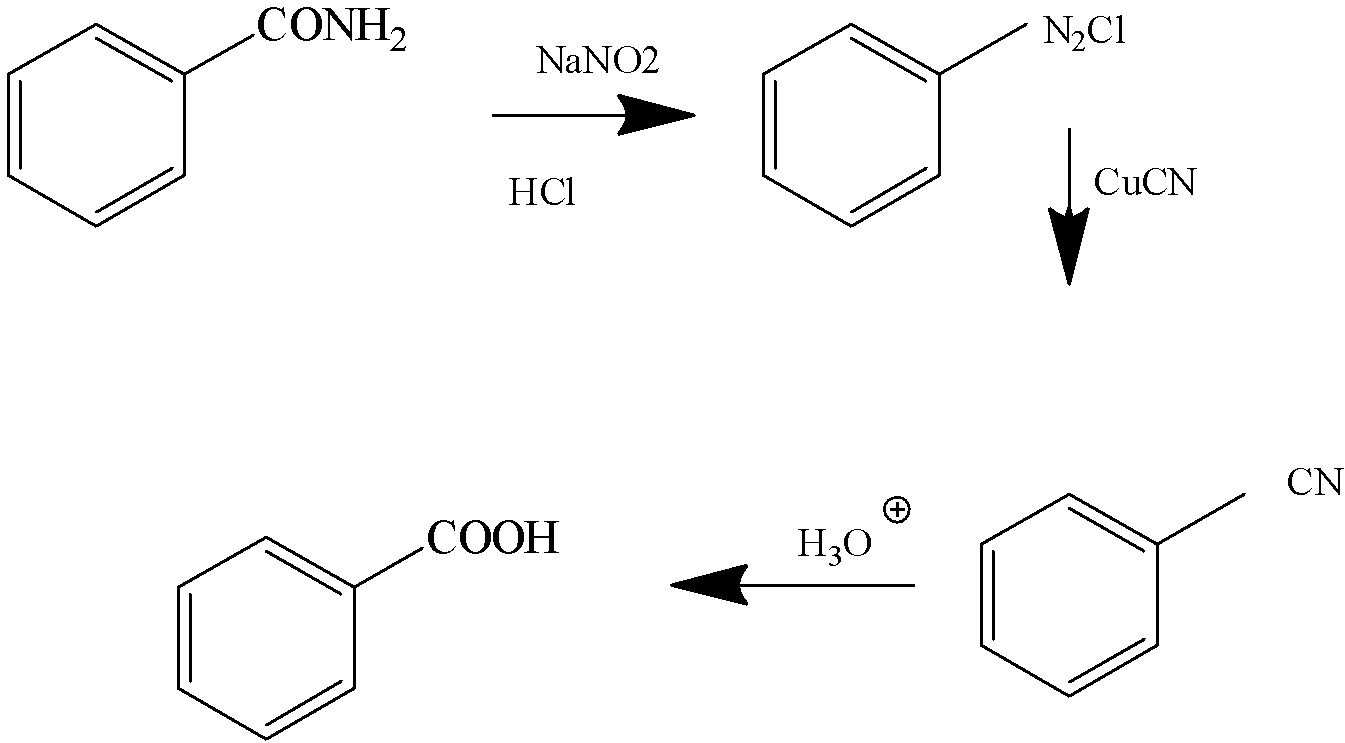
From these reactions we are clear that the compound R is Benzamide, \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CON}}{{\text{H}}_{\text{2}}}\].
So, the correct answer is Option C.
Additional Information:
Amides are a group of chemicals with general formula \[{\text{RCON}}{{\text{H}}_{\text{2}}}\]. Amides are divided into subclasses according to the number of substituents on the nitrogen atom. Benzamide is an aromatic amide having a benzene ring attached to the amide group.
Note: Hoffmann Bromamide reaction is an important organic named reaction. It is used to convert amides into primary amines with one carbon atom less than the parent amide. Also, the diazotization reaction is also an important intermediate reaction in the preparation of a wide variety of organic compounds.
Complete step by step answer:
The compound when heated with \[{\text{aq}}{\text{.NaOH}}\] gives the \[{\text{N}}{{\text{H}}_{\text{3}}}\] gas which means that there is the presence of an -\[{\text{N}}{{\text{H}}_{\text{2}}}\] group.
The reaction with heating the compound with Bromine in \[{\text{aq}}{\text{.NaOH}}\] is the Hoffmann Bromamide reaction. In this reaction the amides are converted to primary amines with one carbon atom less than the parent amide. Here \[{\text{RCON}}{{\text{H}}_{\text{2}}}\] gets converted to \[{\text{RN}}{{\text{H}}_{\text{2}}}\].
And finally, when this \[{\text{RN}}{{\text{H}}_{\text{2}}}\] is treated with \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{/HCl}}\], the diazonium salt of the corresponding compound is formed.
From these reactions we can make sure that the compound contains a- \[{\text{CON}}{{\text{H}}_{\text{2}}}\] group and it is an aromatic compound. Therefore, the compound R will be benzamide. The three reactions can be shown as below:
1.Heating with \[{\text{aq}}{\text{.NaOH}}\]

Benzamide Sodium Benzoate
2.Hoffmann Bromamide Reaction
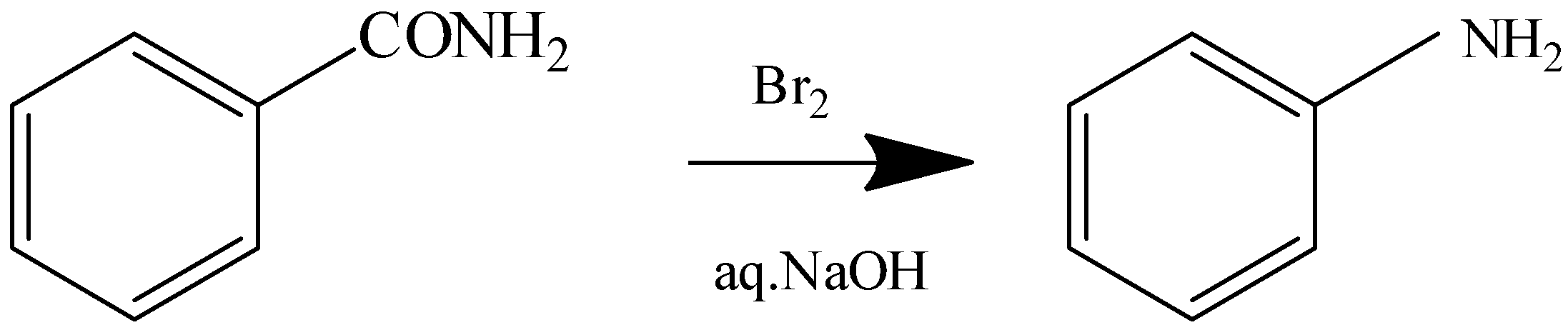
Benzamide Aniline
3.Diazotisation

Benzoic Acid Benzonitrile
From these reactions we are clear that the compound R is Benzamide, \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CON}}{{\text{H}}_{\text{2}}}\].
So, the correct answer is Option C.
Additional Information:
Amides are a group of chemicals with general formula \[{\text{RCON}}{{\text{H}}_{\text{2}}}\]. Amides are divided into subclasses according to the number of substituents on the nitrogen atom. Benzamide is an aromatic amide having a benzene ring attached to the amide group.
Note: Hoffmann Bromamide reaction is an important organic named reaction. It is used to convert amides into primary amines with one carbon atom less than the parent amide. Also, the diazotization reaction is also an important intermediate reaction in the preparation of a wide variety of organic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




