
Complete the following reaction:
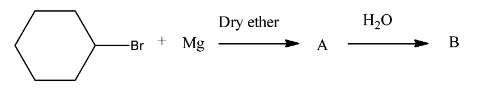

Answer
592.8k+ views
Hint: The given reaction involves the production of Grignard’s reagent as an intermediate. Grignard reagents produce alkanes as a product when allowed to react with water.
Step-by-Step Solution:
Let us first understand what a Grignard reagent is and does before moving on to the solution of this question in particular.
A Grignard reagent is a chemical compound with the general formula of R-Mg-X, where X is a halogen and R is an organic group, normally an alkyl or aryl.
As Example, Methylmagnesium chloride $C{{H}_{3}}MgCl$ and Phenylmagnesium bromide ${{C}_{6}}{{H}_{5}}-MgBr$ are Grignard reagents.
- Grignard compounds are very important reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound R’-X’ in the presence of a suitable catalyst, they typically yield R-R’ and the magnesium halide MgXX’ as a by-product; and the later is insoluble in the solvents normally used. They are similar to organolithium compounds.
- Grignard reagents can be prepared by treating an organic halide with magnesium metal in ether. Either cyclic or acyclic ethers are required to be present in the solution to stabilize the organomagnesium compound.
- Water and air rapidly destroy the reagent by protonolysis or oxidation, are excluded using air-free techniques.
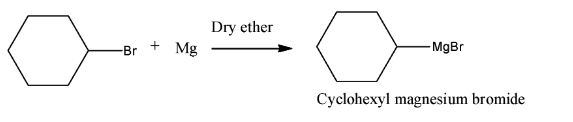
With this idea of synthesis in mind, we can easily solve the first half of the question as we conclude that product A will be cyclohexylmagnesium bromide due to the formation of corresponding Grignard reagent of cyclohexyl bromide.
Now, we know that the reaction of Grignard’s reagent with moisture or water results in the formation of corresponding alkane. Therefore,
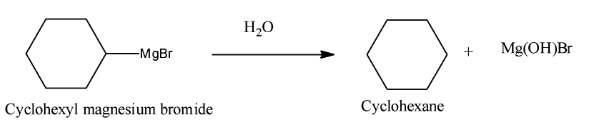
Therefore, we can safely conclude that product A is cyclohexylmagnesium bromide and product B is cyclohexane.
Note: Remember that Grignard reagent on reaction with water will not produce hydroxides but will give alkanes as magnesium is more electropositive and it will attract negative ions more, hence magnesium will form hydroxide. Reactivity of Grignard reagents with water is the reason why we do not use aqueous media to prepare Grignard reagents.
Step-by-Step Solution:
Let us first understand what a Grignard reagent is and does before moving on to the solution of this question in particular.
A Grignard reagent is a chemical compound with the general formula of R-Mg-X, where X is a halogen and R is an organic group, normally an alkyl or aryl.
As Example, Methylmagnesium chloride $C{{H}_{3}}MgCl$ and Phenylmagnesium bromide ${{C}_{6}}{{H}_{5}}-MgBr$ are Grignard reagents.
- Grignard compounds are very important reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound R’-X’ in the presence of a suitable catalyst, they typically yield R-R’ and the magnesium halide MgXX’ as a by-product; and the later is insoluble in the solvents normally used. They are similar to organolithium compounds.
- Grignard reagents can be prepared by treating an organic halide with magnesium metal in ether. Either cyclic or acyclic ethers are required to be present in the solution to stabilize the organomagnesium compound.
- Water and air rapidly destroy the reagent by protonolysis or oxidation, are excluded using air-free techniques.
With this idea of synthesis in mind, we can easily solve the first half of the question as we conclude that product A will be cyclohexylmagnesium bromide due to the formation of corresponding Grignard reagent of cyclohexyl bromide.

Now, we know that the reaction of Grignard’s reagent with moisture or water results in the formation of corresponding alkane. Therefore,

Therefore, we can safely conclude that product A is cyclohexylmagnesium bromide and product B is cyclohexane.
Note: Remember that Grignard reagent on reaction with water will not produce hydroxides but will give alkanes as magnesium is more electropositive and it will attract negative ions more, hence magnesium will form hydroxide. Reactivity of Grignard reagents with water is the reason why we do not use aqueous media to prepare Grignard reagents.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




