
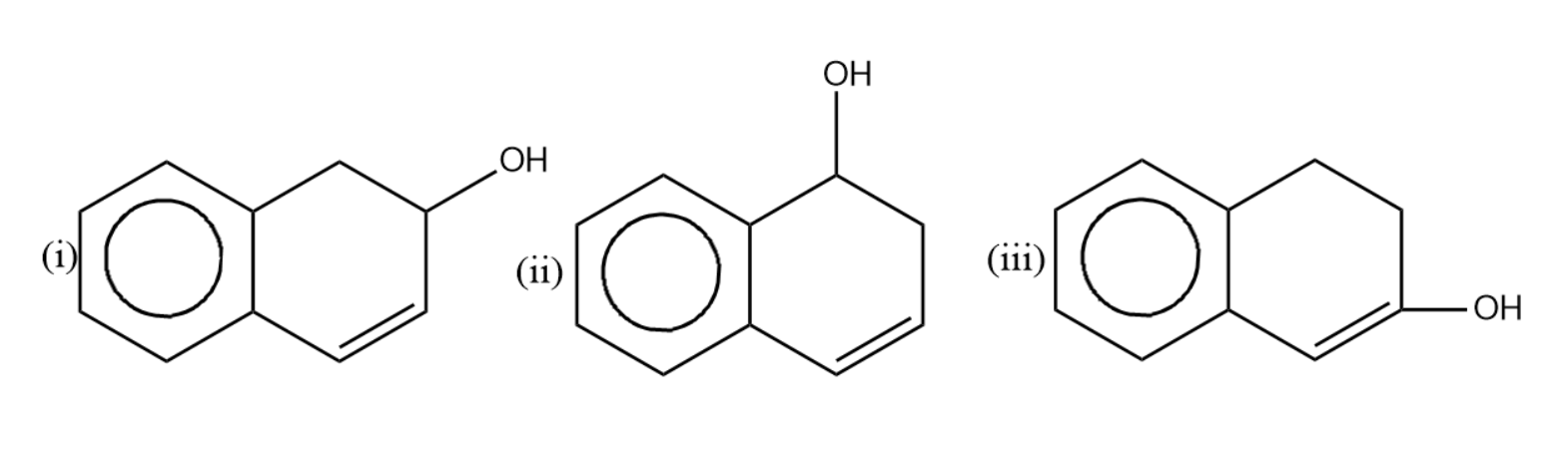
Compare the rate of dehydration of (i), (ii) and (iii) by conc. ${{H}_{2}}S{{O}_{4}}$.
(A) - (i) $>$ (iii) $>$ (ii)
(B)- (i) $>$ (ii) $>$ (iii)
(C)- (ii) $>$ (i) $>$ (iii)
(D)- (ii) $>$ (iii) $>$ (i)

Answer
600k+ views
Hint: Dehydration of alcohols upon reaction with protic acids like ${{H}_{2}}S{{O}_{4}}$ , tends to lose a molecule of water to form alkenes. The rate of dehydration is highest for tertiary alcohols as compared to secondary and primary alcohols. The more the resonating structures formed, the more will be the stability, hence higher will the rate of dehydration.
Complete answer:
-When alcohol undergoes dehydration, it forms alkenes. This is a simple elimination reaction. The rates of dehydration differ as tertiary having the highest rate of dehydration due to the formation of much stable carbocation in tertiary alcohols.
-Dehydration of alcohol is a three-step reaction-
(i) The first step is the formation of protonated alcohol. In this step, the alcohol on treatment with a protic acid acts as a lewis base due to the presence of a single pair present on the oxygen atom. This is a reversible step.
(ii) The second step is the formation of carbocation by making and formation of bonds. In this step, the C-O bond breaks generating a carbocation. This is the slowest and the rate-determining step.
(iii) The third step is the formation of alkene formation. In this step, the proton generated is eliminated with the help of a base. The carbon atom neighboring to the carbocation breaks the present alkane bond to form an alkene.
-Let us now see the dehydration of the given molecules-
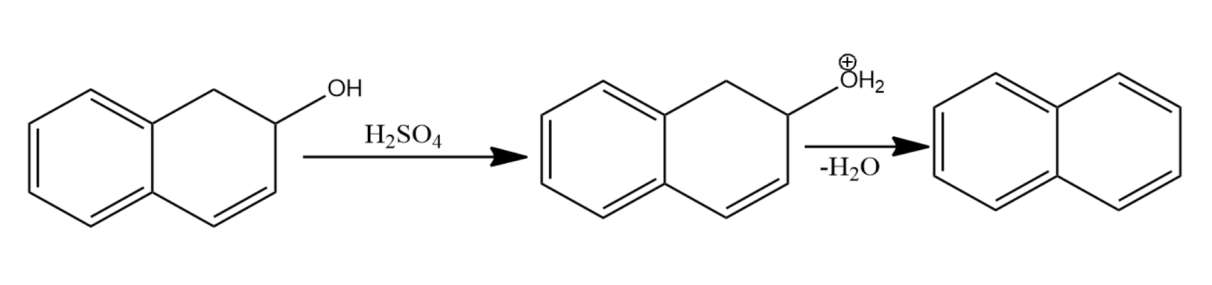
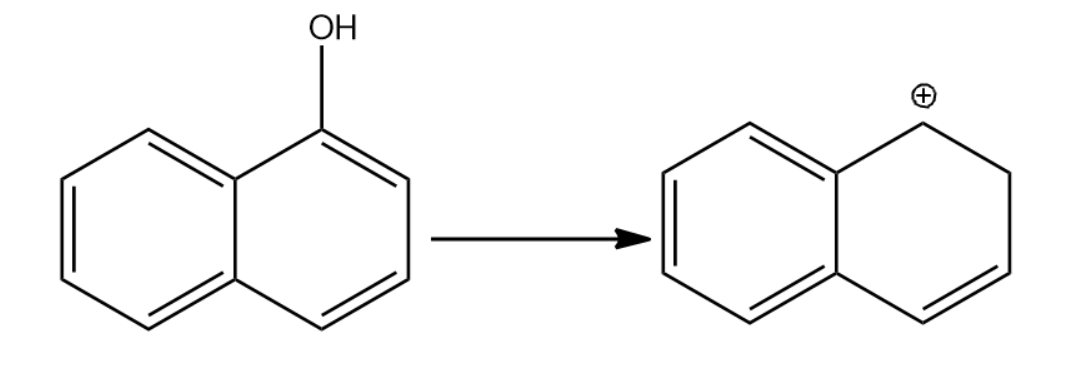
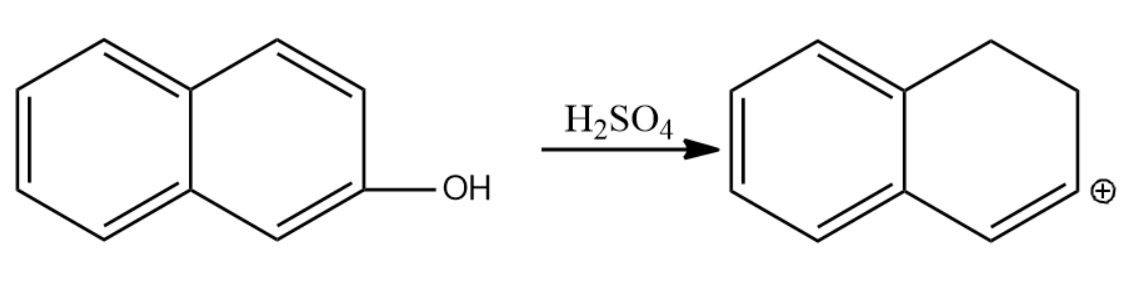
Since the structure (i) on dehydration forms a carbocation which attains stability by resonance with the benzene. Hence it will have seven resonating structures. The carbocation formed in (ii) attains stability by resonating with only one benzene ring, hence it will form six resonating structures. The carbocation formed in (iii) is vinyl carbocation hence it will be least stable.
So the correct rate of dehydration is (i) $>$ (ii) $>$ (iii) .
So, the correct answer is “Option B”
Note: The dehydration of tertiary and secondary alcohol is known as an E1 reaction which is a two-step mechanism. The dehydration of primary alcohol is an E2 reaction which is a single-step mechanism.
Complete answer:
-When alcohol undergoes dehydration, it forms alkenes. This is a simple elimination reaction. The rates of dehydration differ as tertiary having the highest rate of dehydration due to the formation of much stable carbocation in tertiary alcohols.
-Dehydration of alcohol is a three-step reaction-
(i) The first step is the formation of protonated alcohol. In this step, the alcohol on treatment with a protic acid acts as a lewis base due to the presence of a single pair present on the oxygen atom. This is a reversible step.
(ii) The second step is the formation of carbocation by making and formation of bonds. In this step, the C-O bond breaks generating a carbocation. This is the slowest and the rate-determining step.
(iii) The third step is the formation of alkene formation. In this step, the proton generated is eliminated with the help of a base. The carbon atom neighboring to the carbocation breaks the present alkane bond to form an alkene.
-Let us now see the dehydration of the given molecules-



Since the structure (i) on dehydration forms a carbocation which attains stability by resonance with the benzene. Hence it will have seven resonating structures. The carbocation formed in (ii) attains stability by resonating with only one benzene ring, hence it will form six resonating structures. The carbocation formed in (iii) is vinyl carbocation hence it will be least stable.
So the correct rate of dehydration is (i) $>$ (ii) $>$ (iii) .
So, the correct answer is “Option B”
Note: The dehydration of tertiary and secondary alcohol is known as an E1 reaction which is a two-step mechanism. The dehydration of primary alcohol is an E2 reaction which is a single-step mechanism.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




