
$CO_{3}^{2-}$ anions has which of the following characteristics?
This question has multiple correct options
A. bonds of unequal length
B. $s{{p}^{2}}$ hybridization of C atom
C. resonance stabilization
D. same bond angles
Answer
582.6k+ views
Hint: As we know that $CO_{3}^{2-}$ is called carbonate ion. It is the simplest oxocarbon anion, that has one carbon atom which is surrounded by three oxygen atoms. It is found that $CO_{3}^{2-}$ is a polyatomic ion.
Complete answer:
- Let’s discuss about bond lengths of $CO_{3}^{2-}$ ions:
It is found that there are three identical bonds present in $CO_{3}^{2-}$. We can represent the Lewis structure of $CO_{3}^{2-}$ as:
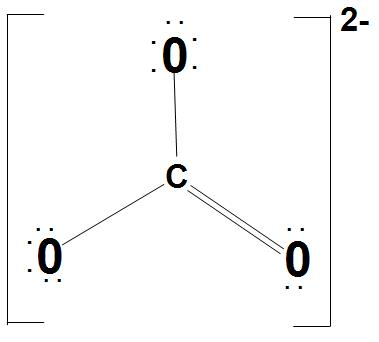
- Let’s discuss about $s{{p}^{2}}$ hybridization of C atom:
It is found that the carbonate ion, carbon is bonded with one oxygen atom by a double bond and with two oxygen by a single bond. It has trigonal planar geometry which clearly mentions that the carbon is $s{{p}^{2}}$ hybridized.
- Let’s discuss resonance stabilization: It is found that the carbonate anion shows resonance stabilization. We can see the resonance structures of carbonate ion with resonance hybrid:
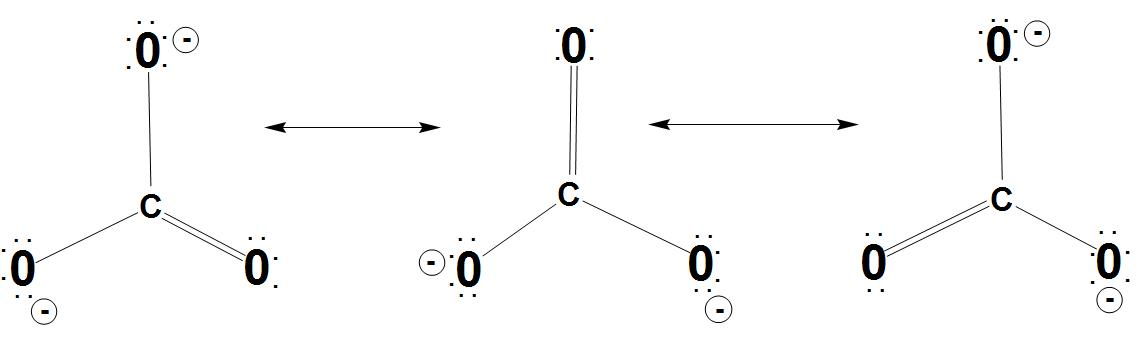
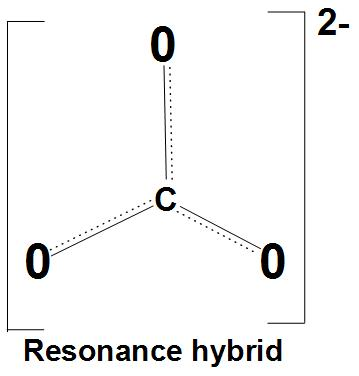
- Let’s discuss about same bond angles:
As we have discussed , the carbonate ion has trigonal planar geometry, just like $B{{F}_{3}}$ , with a bond angle of 120 degree. It is found that carbonate ions have the same bond angles.
- Hence, we can conclude that the correct options are (b), (c), (d) that is $CO_{3}^{2-}$ has $s{{p}^{2}}$ hybridization of C atom, resonance stabilization and same bond angles.
Note: - $CO_{3}^{2-}$ should not be confused with $C{{O}_{3}}$. The main difference between both of these is that $CO_{3}^{2-}$ is carbonate ion, which is a stable oxocarbon anion. Whereas, $C{{O}_{3}}$ is carbon trioxide, which is an unstable oxide of carbon (oxocarbon) .
Complete answer:
- Let’s discuss about bond lengths of $CO_{3}^{2-}$ ions:
It is found that there are three identical bonds present in $CO_{3}^{2-}$. We can represent the Lewis structure of $CO_{3}^{2-}$ as:

- Let’s discuss about $s{{p}^{2}}$ hybridization of C atom:
It is found that the carbonate ion, carbon is bonded with one oxygen atom by a double bond and with two oxygen by a single bond. It has trigonal planar geometry which clearly mentions that the carbon is $s{{p}^{2}}$ hybridized.
- Let’s discuss resonance stabilization: It is found that the carbonate anion shows resonance stabilization. We can see the resonance structures of carbonate ion with resonance hybrid:


- Let’s discuss about same bond angles:
As we have discussed , the carbonate ion has trigonal planar geometry, just like $B{{F}_{3}}$ , with a bond angle of 120 degree. It is found that carbonate ions have the same bond angles.
- Hence, we can conclude that the correct options are (b), (c), (d) that is $CO_{3}^{2-}$ has $s{{p}^{2}}$ hybridization of C atom, resonance stabilization and same bond angles.
Note: - $CO_{3}^{2-}$ should not be confused with $C{{O}_{3}}$. The main difference between both of these is that $CO_{3}^{2-}$ is carbonate ion, which is a stable oxocarbon anion. Whereas, $C{{O}_{3}}$ is carbon trioxide, which is an unstable oxide of carbon (oxocarbon) .
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

How was the Civil Disobedience Movement different from class 12 social science CBSE

How is democracy better than other forms of government class 12 social science CBSE




