
$C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}+CHC{{l}_{3}}+3KOH\to A+B+3{{H}_{2}}O$
Two compounds A and B respectively are:
(a)- $C{{H}_{3}}C{{H}_{2}}CON{{H}_{2}}\text{ and }3KCl$
(b)- ${{C}_{2}}{{H}_{5}}NC\text{ and }{{\text{K}}_{2}}\text{C}{{\text{O}}_{3}}$
(c)- ${{C}_{2}}{{H}_{5}}NC\text{ and 3KCl}$
(d)- ${{C}_{2}}{{H}_{5}}CN\text{ and 3KCl}$
Answer
593.1k+ views
Hint: This reaction is called the carbylamine reaction. This is based on the reaction with electrophiles. Isocyanide is the main product of this reaction.
Complete answer:
Due to the presence of a lone pair of electron on the nitrogen atom, animes like ammonia are good nucleophiles and hence react with a variety of electrophiles (electron-deficient compounds) such as metal ions, alkyl halides, acid chlorides, acid anhydrides, chloroform, etc.
Reaction with chloroform- This reaction is called carbylamine reaction. It is also used as an isocyanide test.
Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH produce isocyanide or carbylamines which have a very unpleasant odor.
The general reaction of carbylamines is shown below:
When primary amine reacts with chloroform forms alkyl isocyanide.
$R-N{{H}_{2}}+CHC{{l}_{3}}+3KOH\to R-N\equiv C+3KCl+3{{H}_{2}}O$
So in the question ethylamine is reacted with chloroform and potassium hydroxide.
Hence, it forms ethyl isocyanide with 3 moles of potassium chloride and 3 moles of water.
The reaction is given below:
$C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}+CHC{{l}_{3}}+3KOH\to C{{H}_{3}}-C{{H}_{2}}N\equiv C+3KCl+3{{H}_{2}}O$
The aromatic primary amines also give this test. When aniline reacts with chloroform and potassium hydroxide it forms phenyl isocyanide with 3 moles of potassium chloride and 3 moles of water.
The reaction is given below:
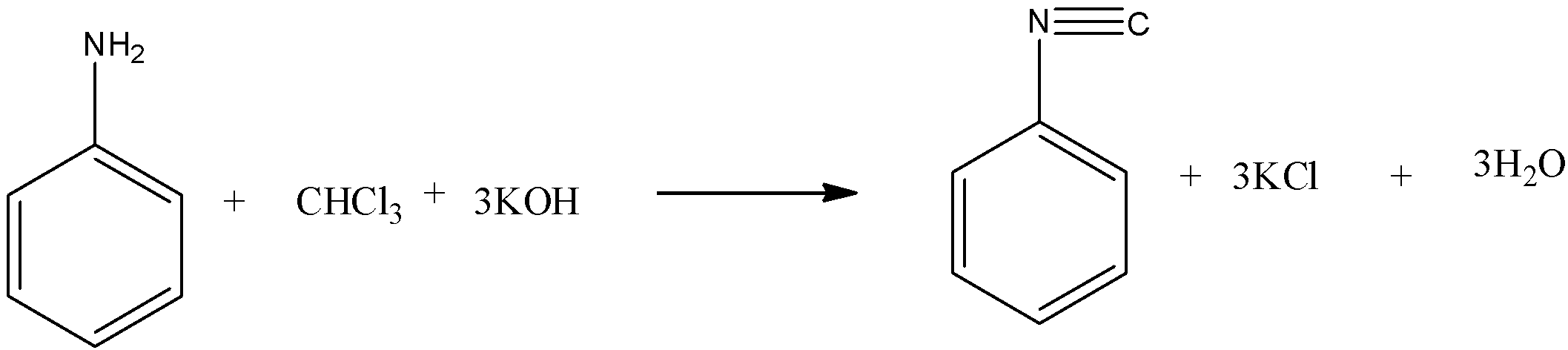
This reaction is not given by secondary and tertiary amines (both aliphatic and aromatic compounds). Therefore, it is used to distinguish primary amines from secondary and tertiary amines.
So, the correct answer is “Option C”.
Note: You may get confused between options (c) and (d) because their formula is the same but the bonding is different. This is a specific reaction that forms the only isocyanide. While writing the reaction by-products should be correct.
Complete answer:
Due to the presence of a lone pair of electron on the nitrogen atom, animes like ammonia are good nucleophiles and hence react with a variety of electrophiles (electron-deficient compounds) such as metal ions, alkyl halides, acid chlorides, acid anhydrides, chloroform, etc.
Reaction with chloroform- This reaction is called carbylamine reaction. It is also used as an isocyanide test.
Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH produce isocyanide or carbylamines which have a very unpleasant odor.
The general reaction of carbylamines is shown below:
When primary amine reacts with chloroform forms alkyl isocyanide.
$R-N{{H}_{2}}+CHC{{l}_{3}}+3KOH\to R-N\equiv C+3KCl+3{{H}_{2}}O$
So in the question ethylamine is reacted with chloroform and potassium hydroxide.
Hence, it forms ethyl isocyanide with 3 moles of potassium chloride and 3 moles of water.
The reaction is given below:
$C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}+CHC{{l}_{3}}+3KOH\to C{{H}_{3}}-C{{H}_{2}}N\equiv C+3KCl+3{{H}_{2}}O$
The aromatic primary amines also give this test. When aniline reacts with chloroform and potassium hydroxide it forms phenyl isocyanide with 3 moles of potassium chloride and 3 moles of water.
The reaction is given below:

This reaction is not given by secondary and tertiary amines (both aliphatic and aromatic compounds). Therefore, it is used to distinguish primary amines from secondary and tertiary amines.
So, the correct answer is “Option C”.
Note: You may get confused between options (c) and (d) because their formula is the same but the bonding is different. This is a specific reaction that forms the only isocyanide. While writing the reaction by-products should be correct.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




