
Cationic surfactants is/are:
(A) ${{C}_{17}}{{H}_{35}}COONa$
(B) ${{C}_{17}}{{H}_{35}}COOK$
(C) ${{C}_{17}}{{H}_{35}}S{{O}_{3}}Na$
(D) ${{C}_{16}}{{H}_{33}}N{{(C{{H}_{3}})}_{3}}Cl$
Answer
573.9k+ views
Hint: Cationic surfactants are classified under the category of surfactants. These consist of a long hydrocarbon chain, and a water-loving end. Now, identify the given options whether they are anionic, or cationic surfactants.
Complete answer:
-First, let us know about the cationic surfactants. These are types of soaps, or detergent, as mentioned falls under the category of surfactants. It consists of a hydrophilic part in the long hydrocarbon chain.
-It contains a positively charged ion, or from the name we can say a cation is attached to the hydrophilic part.
-Now, let us look at the given options, the first is ${{C}_{17}}{{H}_{35}}COONa$, it is named as sodium stearate, considered to be an anionic surfactant, as the functional group present at the head part is anionic in nature.
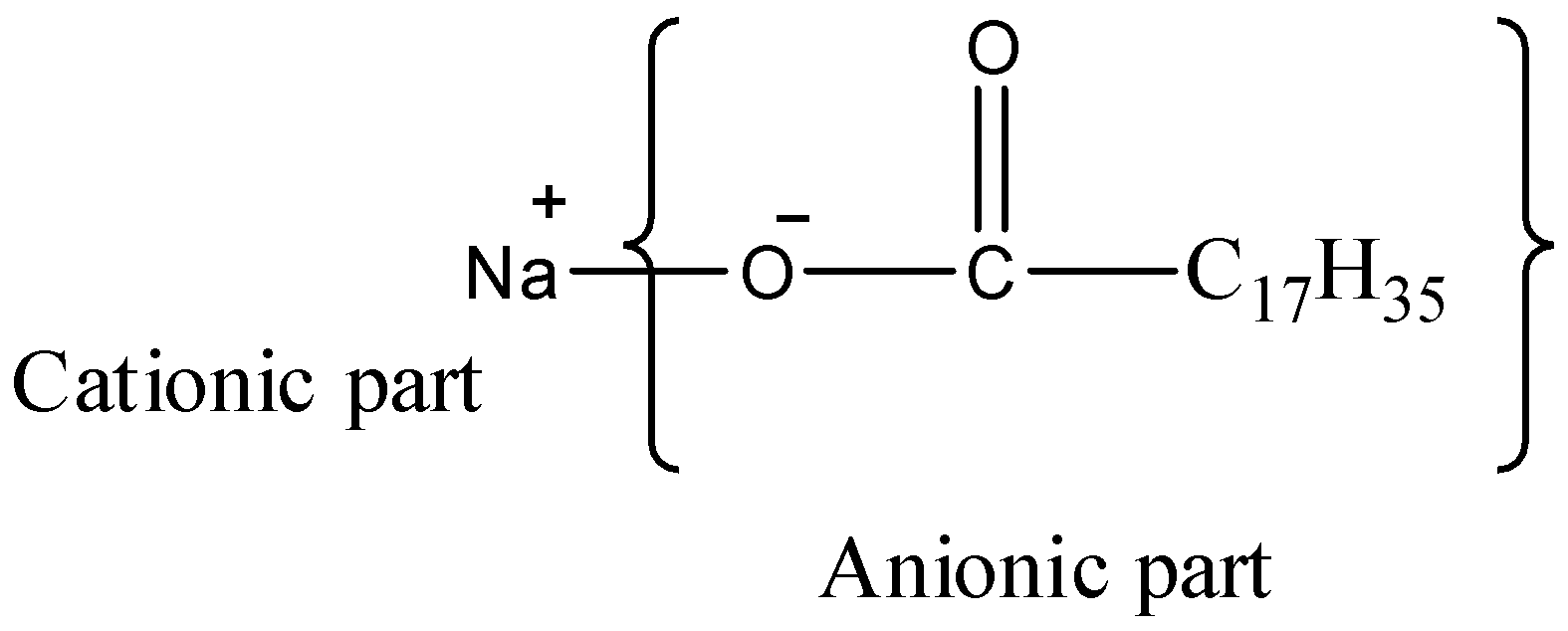
-The second option is ${{C}_{17}}{{H}_{35}}COOK$ , it is named as potassium stearate, and it also falls under the category of anionic surfactants.
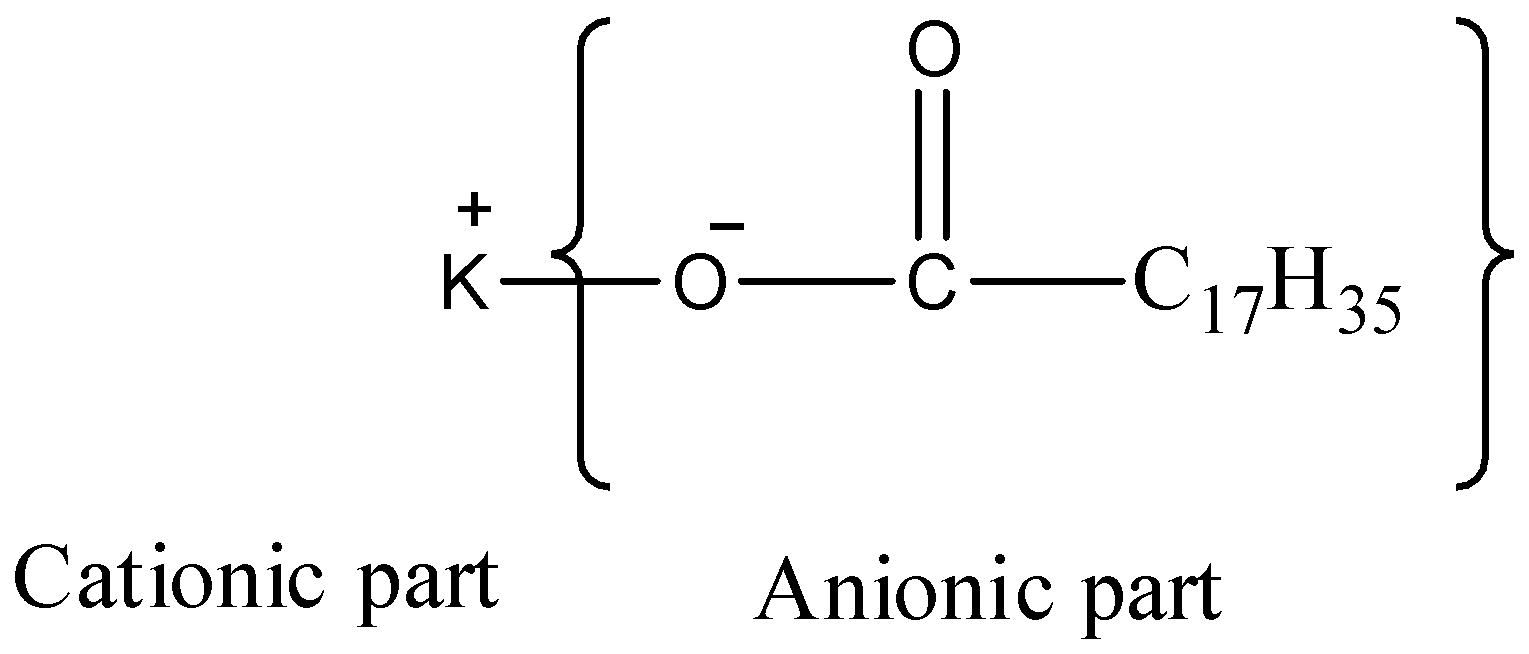
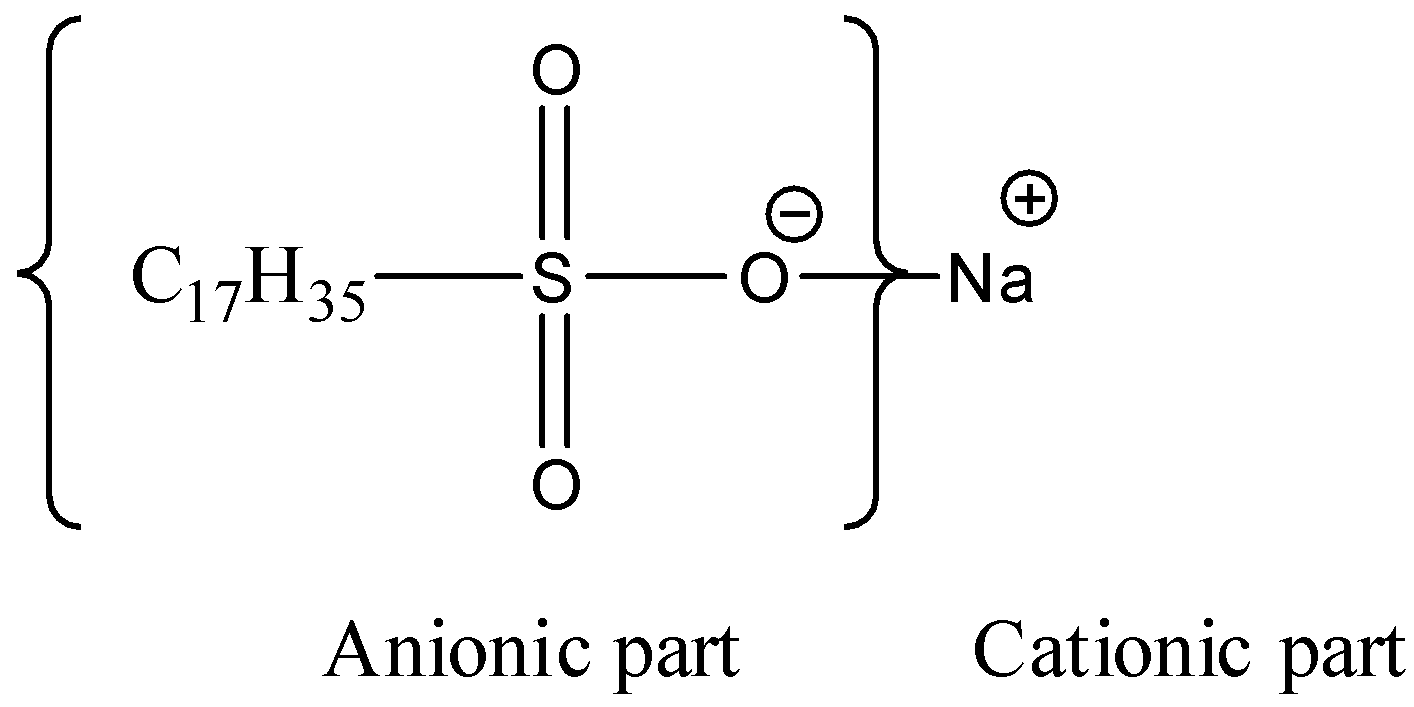
-The third option we have i.e., it is named as sodium decanesulfonate ${{C}_{17}}{{H}_{35}}S{{O}_{3}}Na$, and it is also an anionic surfactant.
-Talking about the fourth option i.e. ${{C}_{16}}{{H}_{33}}N{{(C{{H}_{3}})}_{3}}Cl$ , it is named as cetyl trimethyl ammonium chloride, and it is a cationic surfactant, as it contains a nitrogen atom having positive charge on it.
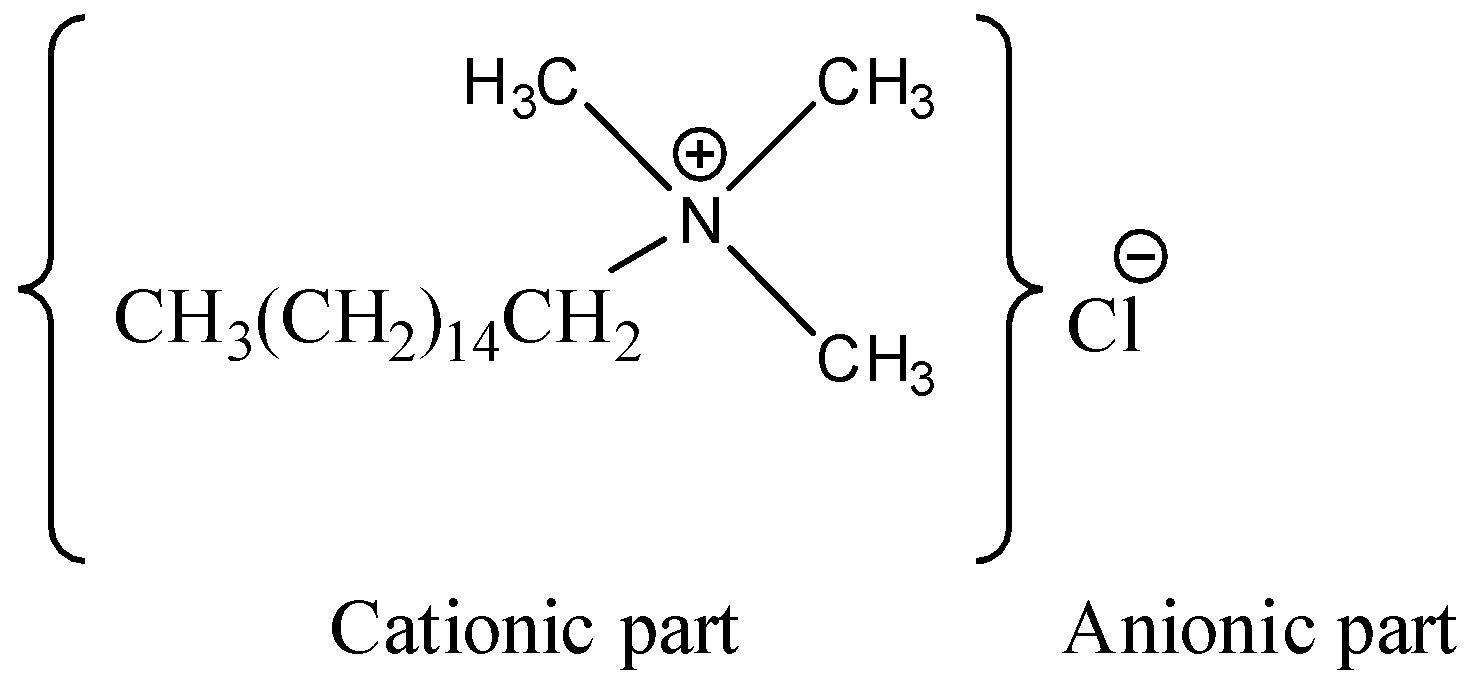
- Thus, in the last we can conclude that the cationic surfactant is ${{C}_{16}}{{H}_{33}}N{{(C{{H}_{3}})}_{3}}Cl$
The correct option is (D).
Note: Don’t get confused between the cationic, and anionic surfactants. We have already discussed the cationic surfactants. Anionic surfactants are those having hydrophobic part, or we can say lipophilic part i.e. water hating end, and an anion attached to it.
Complete answer:
-First, let us know about the cationic surfactants. These are types of soaps, or detergent, as mentioned falls under the category of surfactants. It consists of a hydrophilic part in the long hydrocarbon chain.
-It contains a positively charged ion, or from the name we can say a cation is attached to the hydrophilic part.
-Now, let us look at the given options, the first is ${{C}_{17}}{{H}_{35}}COONa$, it is named as sodium stearate, considered to be an anionic surfactant, as the functional group present at the head part is anionic in nature.

-The second option is ${{C}_{17}}{{H}_{35}}COOK$ , it is named as potassium stearate, and it also falls under the category of anionic surfactants.

-The third option we have i.e., it is named as sodium decanesulfonate ${{C}_{17}}{{H}_{35}}S{{O}_{3}}Na$, and it is also an anionic surfactant.

-Talking about the fourth option i.e. ${{C}_{16}}{{H}_{33}}N{{(C{{H}_{3}})}_{3}}Cl$ , it is named as cetyl trimethyl ammonium chloride, and it is a cationic surfactant, as it contains a nitrogen atom having positive charge on it.

- Thus, in the last we can conclude that the cationic surfactant is ${{C}_{16}}{{H}_{33}}N{{(C{{H}_{3}})}_{3}}Cl$
The correct option is (D).
Note: Don’t get confused between the cationic, and anionic surfactants. We have already discussed the cationic surfactants. Anionic surfactants are those having hydrophobic part, or we can say lipophilic part i.e. water hating end, and an anion attached to it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




