
Carboxylic acids do not give the characteristic reaction of \[{\text{ > C}} = {\text{O}}\] group because of:
(A) polar nature.
(B) resonance.
(C) symmetrical structure.
(D) attached alkyl group.
Answer
582.9k+ views
Hint: When oxygen atom containing a lone pair of electrons is attached to the carbon-oxygen double bond, resonance is possible. This resonance affects the chemical properties of the compounds.
Complete answer:
List the characteristic reactions of aldehydes as shown below:
(1) Aldehydes undergo nucleophilic addition reactions.
(2) Aldehydes undergo either reduction or oxidation reactions.
(3) Reactions due to acidity of alpha hydrogen atoms.
List the characteristic reactions of carboxylic acids as shown below:
(1) Acidity of carboxylic acids due to breaking of bonds in hydroxyl groups.
(2) Reactions that involve breaking the bond between carbonyl carbon atoms and oxygen atoms of hydroxyl groups.
(3) Reactions involving carboxylic functional groups.
(4) The hydrocarbon part undergoes substitution reactions.
Aldehydes and ketones have carbonyl groups. Due to the presence of carbonyl groups, aldehydes and ketones give certain reactions that are characteristics of carbonyl groups.
Carboxylic acids also contain carbonyl groups. But the structure of the carbonyl group in carboxylic acids is different from the carbonyl group in aldehydes and ketones.
Write the structures of the functional groups of aldehydes and ketones as shown below:
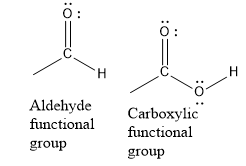
In aldehydes and ketones, the carbonyl group has carbon oxygen double bond. No resonance is present. In carboxylic acids, the carbonyl group has two carbon-oxygen bonds having partial double bond character. This is due to resonance.
Carboxylic acids do not give the characteristic reaction of carbonyl group because of resonance:
Hence, the option (B) resonance is the correct option.
Additional Information: (A) Polar nature: The carbonyl group of carboxylic acids is polar in nature. The carbonyl group of aldehydes and ketones is also polar in nature. Hence, this option is ruled out.
(B) Resonance: The difference behavior of the carbonyl group of carboxylic acid and the carbonyl group of aldehyde and ketones can be attributed to the resonance.
(C) Symmetrical structure: Carboxylate ion has symmetrical structure. But the carboxylic acid group is not symmetrical. But this has nothing to do with characteristic reactions of carbonyl groups.
(D) Attached alkyl group: Alkyl group is attached to carbonyl group of aldehydes, ketones and carboxylic acids. This has nothing to do with the characteristic reactions of carbonyl groups.
Note: The carbonyl group of aldehydes and ketones undergoes nucleophilic addition reactions. The carboxylic functional group of carbonyl compounds undergo substitution reaction.
Complete answer:
List the characteristic reactions of aldehydes as shown below:
(1) Aldehydes undergo nucleophilic addition reactions.
(2) Aldehydes undergo either reduction or oxidation reactions.
(3) Reactions due to acidity of alpha hydrogen atoms.
List the characteristic reactions of carboxylic acids as shown below:
(1) Acidity of carboxylic acids due to breaking of bonds in hydroxyl groups.
(2) Reactions that involve breaking the bond between carbonyl carbon atoms and oxygen atoms of hydroxyl groups.
(3) Reactions involving carboxylic functional groups.
(4) The hydrocarbon part undergoes substitution reactions.
Aldehydes and ketones have carbonyl groups. Due to the presence of carbonyl groups, aldehydes and ketones give certain reactions that are characteristics of carbonyl groups.
Carboxylic acids also contain carbonyl groups. But the structure of the carbonyl group in carboxylic acids is different from the carbonyl group in aldehydes and ketones.
Write the structures of the functional groups of aldehydes and ketones as shown below:

In aldehydes and ketones, the carbonyl group has carbon oxygen double bond. No resonance is present. In carboxylic acids, the carbonyl group has two carbon-oxygen bonds having partial double bond character. This is due to resonance.
Carboxylic acids do not give the characteristic reaction of carbonyl group because of resonance:
Hence, the option (B) resonance is the correct option.
Additional Information: (A) Polar nature: The carbonyl group of carboxylic acids is polar in nature. The carbonyl group of aldehydes and ketones is also polar in nature. Hence, this option is ruled out.
(B) Resonance: The difference behavior of the carbonyl group of carboxylic acid and the carbonyl group of aldehyde and ketones can be attributed to the resonance.
(C) Symmetrical structure: Carboxylate ion has symmetrical structure. But the carboxylic acid group is not symmetrical. But this has nothing to do with characteristic reactions of carbonyl groups.
(D) Attached alkyl group: Alkyl group is attached to carbonyl group of aldehydes, ketones and carboxylic acids. This has nothing to do with the characteristic reactions of carbonyl groups.
Note: The carbonyl group of aldehydes and ketones undergoes nucleophilic addition reactions. The carboxylic functional group of carbonyl compounds undergo substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




