
Carbon donor ligands are strong ligands and usually form low spin complexes. Among the complexes given below, select the high spin complexes.
A.)${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 3- }$
B.)${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 4- }$
C.)${ \left[ Fe{ \left( { C }_{ 2 }{ O }_{ 4 } \right) }_{ 3 } \right] }^{ 3- }$
D.) None of these
Answer
593.1k+ views
Hint: High spin complexes are those which have more spin than that possessed by the parent metal. ${ CN }^{ - }$ are strong field ligands as compared to ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$. In ${ CN }^{ - }$, the electrons mostly pair up while in the case of ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$- , the pairing of electrons is comparatively less.
Complete answer:
Carbon donor ligands usually form low spin complexes, which means that the spin of the resulting complex will be less than that of the parent metal.
Among the given options, ${ \left[ Fe{ \left( { C }_{ 2 }{ O }_{ 4 } \right) }_{ 3 } \right] }^{ 3- }$ is the only high spin complex, it is because the ligand ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$is a weak field donor ligand as compared to ${ CN }^{ - }$ ligand.
In the case of ${ CN }^{ - }$ ligands, they pair up as mostly they form inner ‘d’ orbital complexes.
The electrons in ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$ do not pair up as they do in CN- ligands.
In the case of high spin complexes, their spin magnetic moment increases which is given by the formula :
$spin\quad only\quad magnetic\quad moment=\sqrt { n\left( n+2 \right) } $
Where ‘n’ is the number of unpaired electrons.
So, the correct answer is “Option C”.
Additional Information:
Now, we can look at how to determine the high spin and low spin complexes:
(i) By determining the shape of the comlex .For Eg: tetrahedral, octahedral shape etc.
(ii) By determining the oxidation state of the central metal atom . For example : In ${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 3- }$, the oxidation state of central metal atom Fe is +3.
(iii) By determining the ‘d’ electron configuration of the central metal atom . For Eg: in${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 3- }$, the hybridization is ${ d }^{ 2 }{ sp }^{ 3 }$ , that means the complex forms an inner ‘d’ orbital complex.
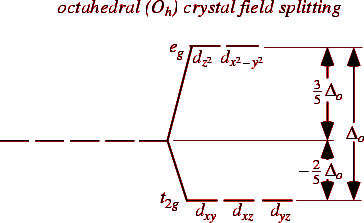
(iii) By drawing the crystal field diagram of the complex with respect to the geometry of the complex.
(iv) By determining whether the splitting energy is greater than the pairing energy.
(v) By determining the strength of the ligand (i.e.by using the help of spectrochemical series)
Crystal field theory (CFT):
Proposed by Bethe and Vavleck.
CFT considers metal- ligand bond as ionic bond, metal acts as the positive point charge and ligands as the negative point charge.
When the ligands approach the central metal, the degenerate ‘d’ orbitals of the metal may undergo splitting , which is called crystal field splitting.
The splitting pattern depends on geometry of the element.
Note:
Crystal field splitting is not a perfect theory. It also has limitations. For Eg: The CFT considers metal-ligand bond as ionic bond. But the theory depended on geometry which is a contradiction.
Complete answer:
Carbon donor ligands usually form low spin complexes, which means that the spin of the resulting complex will be less than that of the parent metal.
Among the given options, ${ \left[ Fe{ \left( { C }_{ 2 }{ O }_{ 4 } \right) }_{ 3 } \right] }^{ 3- }$ is the only high spin complex, it is because the ligand ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$is a weak field donor ligand as compared to ${ CN }^{ - }$ ligand.
In the case of ${ CN }^{ - }$ ligands, they pair up as mostly they form inner ‘d’ orbital complexes.
The electrons in ${ C }_{ 2 }{ O }_{ 4 }^{ 2- }$ do not pair up as they do in CN- ligands.
In the case of high spin complexes, their spin magnetic moment increases which is given by the formula :
$spin\quad only\quad magnetic\quad moment=\sqrt { n\left( n+2 \right) } $
Where ‘n’ is the number of unpaired electrons.
So, the correct answer is “Option C”.
Additional Information:
Now, we can look at how to determine the high spin and low spin complexes:
(i) By determining the shape of the comlex .For Eg: tetrahedral, octahedral shape etc.
(ii) By determining the oxidation state of the central metal atom . For example : In ${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 3- }$, the oxidation state of central metal atom Fe is +3.
(iii) By determining the ‘d’ electron configuration of the central metal atom . For Eg: in${ \left[ Fe{ \left( CN \right) }_{ 6 } \right] }^{ 3- }$, the hybridization is ${ d }^{ 2 }{ sp }^{ 3 }$ , that means the complex forms an inner ‘d’ orbital complex.
(iii) By drawing the crystal field diagram of the complex with respect to the geometry of the complex.
(iv) By determining whether the splitting energy is greater than the pairing energy.
(v) By determining the strength of the ligand (i.e.by using the help of spectrochemical series)
Crystal field theory (CFT):
Proposed by Bethe and Vavleck.
CFT considers metal- ligand bond as ionic bond, metal acts as the positive point charge and ligands as the negative point charge.
When the ligands approach the central metal, the degenerate ‘d’ orbitals of the metal may undergo splitting , which is called crystal field splitting.
The splitting pattern depends on geometry of the element.

Note:
Crystal field splitting is not a perfect theory. It also has limitations. For Eg: The CFT considers metal-ligand bond as ionic bond. But the theory depended on geometry which is a contradiction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




