
Calculate the fractional void volumes in the c.c.p. and h.c.p.structures of hard spheres.
Answer
573.9k+ views
Hint: In a unit cell, the space which is not occupied by the constituent particles in the unit cell is known as the void space. If we consider the total volume of a unit cell as 1.then the fraction of void space would be equal to,
$\text{ The fraction of void space = 1}-\text{Packing fraction }$
The $\text{ }{\scriptstyle{}^{0}/{}_{0}}\text{ }$ void space in a unit cell is equal to,
$\text{ }{\scriptstyle{}^{0}/{}_{0}}\text{ Void space = 100}-\text{Packing efficiency }$
Complete step by step answer:
The void fraction is the total fraction of the crystal which is empty. The void fraction can be calculated by subtracting the packing fraction from the volume of the crystal.
The cubic centred crystal or the face-centred cubic crystal structure has 8 atoms at the 8 corners of the cubic structure. The crystal structure contains 6 atoms at the 6 faces of the cubic structure. Thus total number of atoms per unit cubic structure is equal to,
$\begin{align}
& \text{ No}\text{. of atom = 8 }\times \text{ }\dfrac{1}{8}\text{ contribution at corner + 6 }\times \dfrac{1}{2}\text{ contribution at face} \\
& \therefore \text{No}\text{. of atom = 1 + 3 = 4 } \\
\end{align}$
Thus, the CCP contains a total of 4 atoms per unit cell.
The total value of the cubic structure is equal to, $\text{ Volume of unit cell = (edge}{{\text{)}}^{\text{3}}}\text{ = }{{a}^{3}}\text{ }$
Atoms are considered spherical in shape. Thus the volume of an atom is given as,
$\text{ Volume of atom = }\dfrac{4}{3}\text{ }\pi {{\text{r}}^{\text{3}}}\text{ }$
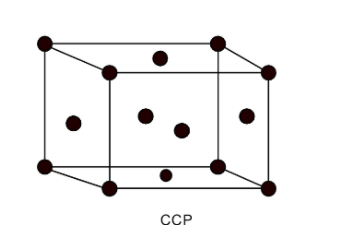
The packing fraction of a unit cell is equal to the amount of the total unit cell occupied by the atoms. Thus packing fraction is a ratio of the volume occupied by the atoms in the cell to the total volume of the unit cell. It is given as follows,
$\text{ Packing fraction of C}\text{.C}\text{.P}\text{. = }\dfrac{\text{total volume occupied by atoms}}{\text{Total volume of unit cell}}\text{ = }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{{{a}^{3}}}\text{ }$
For CCP structure the relationship between the edge length and radius is given as,
$\text{ }\sqrt{2a}\text{ = 4r }\Rightarrow \text{ }a\text{ = 2}\sqrt{2}\text{ r }$
Substitute the values in fraction. We have,
$\text{ P}\text{.}{{\text{F}}_{\text{C}\text{.C}\text{.P}\text{. }}}\text{= }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{{{\left( 2\sqrt{2}\text{ r} \right)}^{3}}}\text{ = }\dfrac{16\pi }{3\times 16\sqrt{2}}\text{ = }\dfrac{\pi }{3\sqrt{2}}\text{ = 0}\text{.74 }$
Thus packing fraction for CCP is equal to $\text{ 0}\text{.74 }$ .the void fraction would be equal to,
$\text{ Void fraction = 1}-\text{Packing fraction = 1}-0.74\text{ = 0}\text{.26 }$
Therefore, the void fraction for CCP is equal to $\text{ 0}\text{.26 }$ .
2) HCP:
The HCP unit cell contains the 6 atoms in the unit cell. The packing fraction of HCP unit cell is given as, $\text{ Packing fraction of H}\text{.C}\text{.P}\text{. = }\dfrac{\text{total volume occupied by atoms}}{\text{Total volume of unit cell}}\text{ = }\dfrac{6\times \dfrac{4}{3}\pi {{r}^{3}}}{{{a}^{3}}}\text{ }$
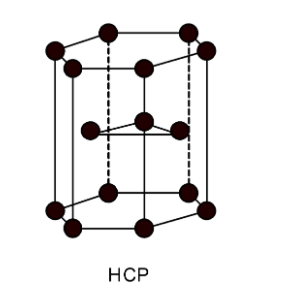
Substitute the values in packing fraction. We have,
$\text{ P}\text{.}{{\text{F}}_{\text{C}\text{.C}\text{.P}\text{. }}}\text{= }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{24\sqrt{2}\text{ }{{\text{r}}^{3}}}\text{ = }\dfrac{8\pi {{\text{r}}^{\text{3}}}}{24\sqrt{2}\text{ }{{\text{r}}^{3}}}\text{ = }\dfrac{\pi }{3\sqrt{2}}\text{ = 0}\text{.74 }$
Thus packing fraction for HCP is equal to $\text{ 0}\text{.74 }$ .the void fraction would be equal to,
$\text{ Void fraction = 1}-\text{Packing fraction = 1}-0.74\text{ = 0}\text{.26 }$
Therefore, the void fraction for HCP is equal to $\text{ 0}\text{.26 }$ .
Thus, the void fraction for CCP is equal to $\text{ 0}\text{.26 }$and for HCP is equal to $\text{ 0}\text{.26 }$.
Note: Note that, if you know the unit cell dimension ‘a’ it is possible to calculate the volume of the unit cell.in calculating the packing fraction, we consider that the atom is a spherical. Remember that, whatever be the constituent in the unit cell (like an atom, molecule, or ions) they are always packed in such a way that there is a free space in the form of the void. For a simple cubic structure, the void fraction is equal to $\text{ 0}\text{.476 }$ and for BCC structure is $\text{ 0}\text{.32 }$ .
$\text{ The fraction of void space = 1}-\text{Packing fraction }$
The $\text{ }{\scriptstyle{}^{0}/{}_{0}}\text{ }$ void space in a unit cell is equal to,
$\text{ }{\scriptstyle{}^{0}/{}_{0}}\text{ Void space = 100}-\text{Packing efficiency }$
Complete step by step answer:
The void fraction is the total fraction of the crystal which is empty. The void fraction can be calculated by subtracting the packing fraction from the volume of the crystal.
The cubic centred crystal or the face-centred cubic crystal structure has 8 atoms at the 8 corners of the cubic structure. The crystal structure contains 6 atoms at the 6 faces of the cubic structure. Thus total number of atoms per unit cubic structure is equal to,
$\begin{align}
& \text{ No}\text{. of atom = 8 }\times \text{ }\dfrac{1}{8}\text{ contribution at corner + 6 }\times \dfrac{1}{2}\text{ contribution at face} \\
& \therefore \text{No}\text{. of atom = 1 + 3 = 4 } \\
\end{align}$
Thus, the CCP contains a total of 4 atoms per unit cell.
The total value of the cubic structure is equal to, $\text{ Volume of unit cell = (edge}{{\text{)}}^{\text{3}}}\text{ = }{{a}^{3}}\text{ }$
Atoms are considered spherical in shape. Thus the volume of an atom is given as,
$\text{ Volume of atom = }\dfrac{4}{3}\text{ }\pi {{\text{r}}^{\text{3}}}\text{ }$

The packing fraction of a unit cell is equal to the amount of the total unit cell occupied by the atoms. Thus packing fraction is a ratio of the volume occupied by the atoms in the cell to the total volume of the unit cell. It is given as follows,
$\text{ Packing fraction of C}\text{.C}\text{.P}\text{. = }\dfrac{\text{total volume occupied by atoms}}{\text{Total volume of unit cell}}\text{ = }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{{{a}^{3}}}\text{ }$
For CCP structure the relationship between the edge length and radius is given as,
$\text{ }\sqrt{2a}\text{ = 4r }\Rightarrow \text{ }a\text{ = 2}\sqrt{2}\text{ r }$
Substitute the values in fraction. We have,
$\text{ P}\text{.}{{\text{F}}_{\text{C}\text{.C}\text{.P}\text{. }}}\text{= }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{{{\left( 2\sqrt{2}\text{ r} \right)}^{3}}}\text{ = }\dfrac{16\pi }{3\times 16\sqrt{2}}\text{ = }\dfrac{\pi }{3\sqrt{2}}\text{ = 0}\text{.74 }$
Thus packing fraction for CCP is equal to $\text{ 0}\text{.74 }$ .the void fraction would be equal to,
$\text{ Void fraction = 1}-\text{Packing fraction = 1}-0.74\text{ = 0}\text{.26 }$
Therefore, the void fraction for CCP is equal to $\text{ 0}\text{.26 }$ .
2) HCP:
The HCP unit cell contains the 6 atoms in the unit cell. The packing fraction of HCP unit cell is given as, $\text{ Packing fraction of H}\text{.C}\text{.P}\text{. = }\dfrac{\text{total volume occupied by atoms}}{\text{Total volume of unit cell}}\text{ = }\dfrac{6\times \dfrac{4}{3}\pi {{r}^{3}}}{{{a}^{3}}}\text{ }$

Substitute the values in packing fraction. We have,
$\text{ P}\text{.}{{\text{F}}_{\text{C}\text{.C}\text{.P}\text{. }}}\text{= }\dfrac{4\times \dfrac{4}{3}\pi {{r}^{3}}}{24\sqrt{2}\text{ }{{\text{r}}^{3}}}\text{ = }\dfrac{8\pi {{\text{r}}^{\text{3}}}}{24\sqrt{2}\text{ }{{\text{r}}^{3}}}\text{ = }\dfrac{\pi }{3\sqrt{2}}\text{ = 0}\text{.74 }$
Thus packing fraction for HCP is equal to $\text{ 0}\text{.74 }$ .the void fraction would be equal to,
$\text{ Void fraction = 1}-\text{Packing fraction = 1}-0.74\text{ = 0}\text{.26 }$
Therefore, the void fraction for HCP is equal to $\text{ 0}\text{.26 }$ .
Thus, the void fraction for CCP is equal to $\text{ 0}\text{.26 }$and for HCP is equal to $\text{ 0}\text{.26 }$.
Note: Note that, if you know the unit cell dimension ‘a’ it is possible to calculate the volume of the unit cell.in calculating the packing fraction, we consider that the atom is a spherical. Remember that, whatever be the constituent in the unit cell (like an atom, molecule, or ions) they are always packed in such a way that there is a free space in the form of the void. For a simple cubic structure, the void fraction is equal to $\text{ 0}\text{.476 }$ and for BCC structure is $\text{ 0}\text{.32 }$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




