
But-2-en-1-ol is the IUPAC name of:
A) $\text{ C}{{\text{H}}_{\text{3}}}-\text{CH=CH}-\text{CHO}$
B) $\text{ C}{{\text{H}}_{\text{2}}}\text{=CH}-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{2}}}-\text{OH}$
C) $\text{ C}{{\text{H}}_{\text{3}}}-\text{CH=CH}-\text{OH}$
D) $\text{ C}{{\text{H}}_{\text{3}}}-\text{CH=CH}-\text{C}{{\text{H}}_{\text{2}}}-\text{OH }$
Answer
565.5k+ views
Hint: The International Union of Pure and Applied Chemistry (IUPAC) has set a rule to determine the nomenclature of the compounds.
The complete IUPAC name of an organic compound may be represented as:
$\text{ Prefix + Word root + Primary suffix + Secondary suffix }$
The functional groups in the organic compounds are treated as the secondary suffix, while the linkage between the carbon bonds is the primary suffix. The alcohol is the functional group$\text{ (}-\text{OH) }$. The numbering is always done in such a way that the functional groups get the lower position and others are numbered with respect to it.
Complete answer:
According to which the name of the compounds consists of three parts:
i) Word root
ii) Suffix
iii) Prefix
The general representation of the name of an organic compound is shown as follows:
$\text{ Prefix + Word root + Primary suffix + Secondary suffix }$
Here, the prefix is the substituents in the organic compounds .These are named as the position followed by the name of it.
The word root is the total number of carbon atoms in the longest chain. This is the number of carbon in the parent chain.
The primary suffix represents the linkage in the carbon atoms. For single linkage, it is –ane for the double bond it is –ene and for a triple bond, it is –yne. This primary suffix is followed by the position of the carbon acquiring multiple bonds.
The secondary suffix is the functional groups present in the molecule. It is added just after the primary suffix. Each functional group has its suffix which is to be added along with the position of it.
Here, we have given the compounds But-2-en-1-ol.
Let's find out the structure of the compound.
Here, the compounds have no substituents as the prefix is not present.
The word root is ‘but’, which means the compound contains the longest chain of carbon which has 4 carbons in it. the skeleton for word root ‘but ‘ is as shown below,
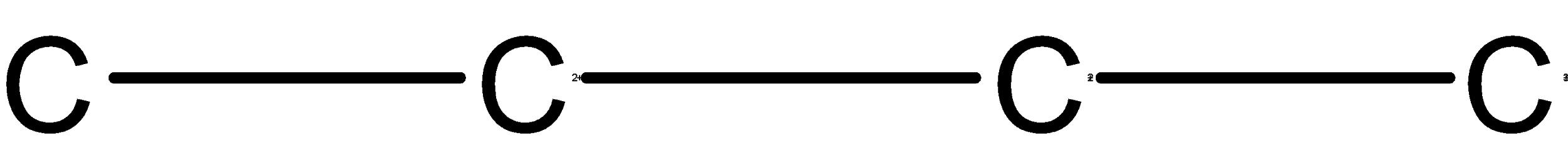
The given IUPAC name has the primary suffix as ‘ene’.The double bond is at the 2 position carbon .thus the but-2-ene is as shown below,
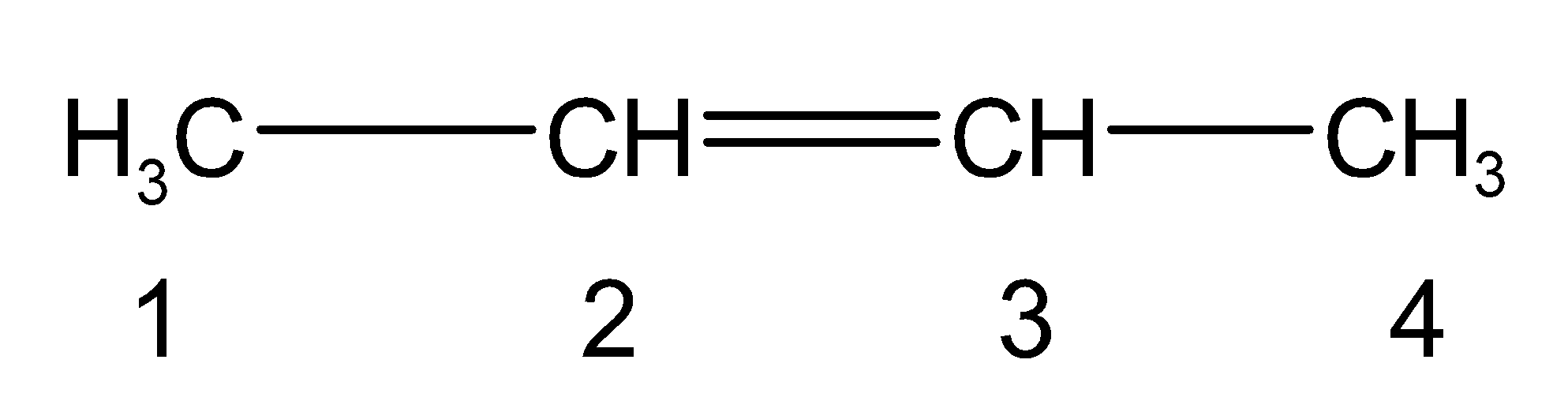
Don’t forget, the compound does have the secondary suffix or the functional group at position 1.It is written as ‘-ol’.Thus the molecule has alcohol $\text{ (}-\text{OH) }$ as the functional group. It is at the first carbon. The carbon double bond should be located at the second carbon for the alcohol. Thus, the complete structure of But-2-en-1-ol is as follows,
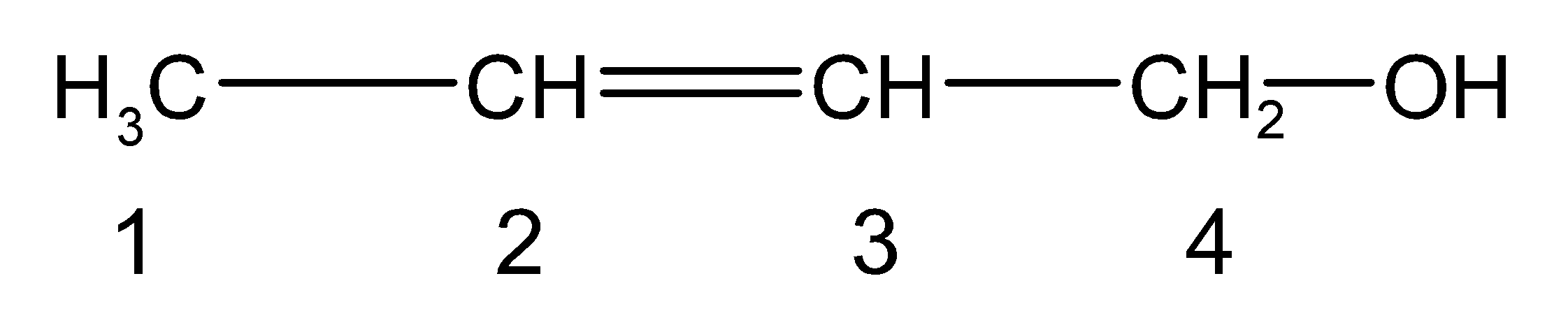
The structure can be written in the condensed formula as follows,
$\text{ C}{{\text{H}}_{\text{3}}}-\text{CH=CH}-\text{C}{{\text{H}}_{\text{2}}}-\text{OH }$
Hence, (D) is the correct option.
Note:
It may be noted that, while adding the secondary suffix to the primary suffix, the terminal ‘e’ of the primary suffix (i.e. ane, ene, or yne) is dropped if the secondary suffix begins with the vowel (i.e. a, e, i, o, u). However, the terminal –e is retained if the secondary suffix begins with a consonant (other than vowel).
Here, alcohol starts with a vowel thus ‘e’ in the terminal is dropped.
The complete IUPAC name of an organic compound may be represented as:
$\text{ Prefix + Word root + Primary suffix + Secondary suffix }$
The functional groups in the organic compounds are treated as the secondary suffix, while the linkage between the carbon bonds is the primary suffix. The alcohol is the functional group$\text{ (}-\text{OH) }$. The numbering is always done in such a way that the functional groups get the lower position and others are numbered with respect to it.
Complete answer:
According to which the name of the compounds consists of three parts:
i) Word root
ii) Suffix
iii) Prefix
The general representation of the name of an organic compound is shown as follows:
$\text{ Prefix + Word root + Primary suffix + Secondary suffix }$
Here, the prefix is the substituents in the organic compounds .These are named as the position followed by the name of it.
The word root is the total number of carbon atoms in the longest chain. This is the number of carbon in the parent chain.
The primary suffix represents the linkage in the carbon atoms. For single linkage, it is –ane for the double bond it is –ene and for a triple bond, it is –yne. This primary suffix is followed by the position of the carbon acquiring multiple bonds.
The secondary suffix is the functional groups present in the molecule. It is added just after the primary suffix. Each functional group has its suffix which is to be added along with the position of it.
Here, we have given the compounds But-2-en-1-ol.
Let's find out the structure of the compound.
Here, the compounds have no substituents as the prefix is not present.
The word root is ‘but’, which means the compound contains the longest chain of carbon which has 4 carbons in it. the skeleton for word root ‘but ‘ is as shown below,
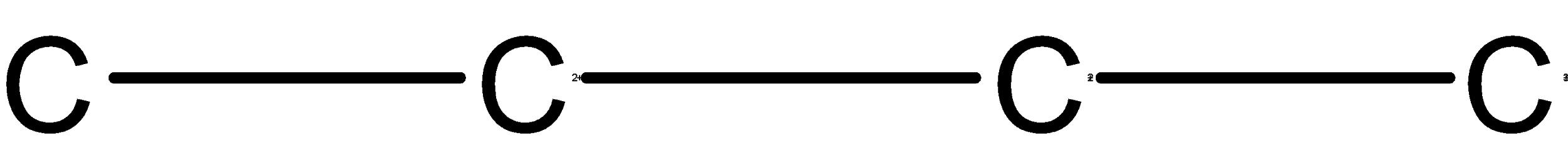
The given IUPAC name has the primary suffix as ‘ene’.The double bond is at the 2 position carbon .thus the but-2-ene is as shown below,
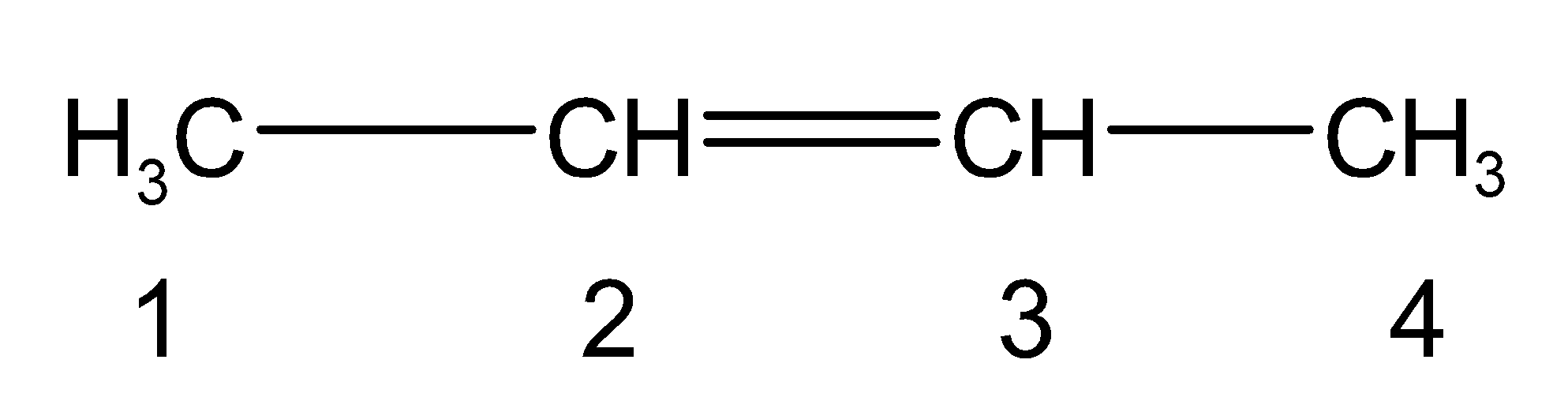
Don’t forget, the compound does have the secondary suffix or the functional group at position 1.It is written as ‘-ol’.Thus the molecule has alcohol $\text{ (}-\text{OH) }$ as the functional group. It is at the first carbon. The carbon double bond should be located at the second carbon for the alcohol. Thus, the complete structure of But-2-en-1-ol is as follows,

The structure can be written in the condensed formula as follows,
$\text{ C}{{\text{H}}_{\text{3}}}-\text{CH=CH}-\text{C}{{\text{H}}_{\text{2}}}-\text{OH }$
Hence, (D) is the correct option.
Note:
It may be noted that, while adding the secondary suffix to the primary suffix, the terminal ‘e’ of the primary suffix (i.e. ane, ene, or yne) is dropped if the secondary suffix begins with the vowel (i.e. a, e, i, o, u). However, the terminal –e is retained if the secondary suffix begins with a consonant (other than vowel).
Here, alcohol starts with a vowel thus ‘e’ in the terminal is dropped.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




