
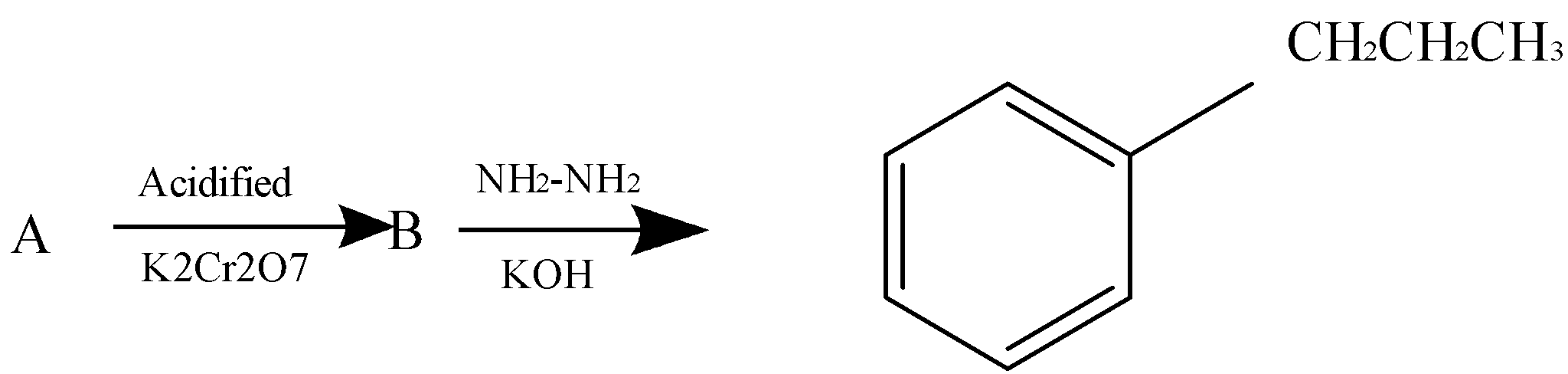
B forms a yellow precipitate on heating with aqueous $\,NaOH\,$ and iodine. Identify A from the above sequence of reaction.
A.$3 - $phenyl propanal
B.$2 - $phenyl propanal
C.$1 - $phenyl propanone
D.$1 - $phenyl propan$ - 2 - $ol

Answer
583.2k+ views
Hint: There are three reactions involved in the sequence.
-Reaction $\,A \to B\,$ involves oxidation of secondary alcohols to ketones in the presence of acidified potassium dichromate.
-Reaction $\,B \to C\,$ involves the Wolff Kishner reduction mechanism where the ketone is converted to corresponding alkane.
-The reaction of B with aqueous $\,NaOH\,$ and iodine is the iodoform reaction which produces a yellow precipitate.
Complete step by step answer:
Iodoform reaction
All compounds containing the $C{H_3}C = O$ group or the $C{H_3}CH(OH)$ group give a positive result with the iodoform test. When iodine and sodium hydroxide are added to a compound containing one of these groups, a pale yellow precipitate of iodoform is further formed.
The iodoform test can be used to identify aldehydes and ketones.
The iodoform test can also be used to differentiate between the alcohols. All secondary alcohols give a positive result since they are easily oxidized to ketones.
Oxidation of Secondary alcohols
Secondary alcohols are oxidized to ketones. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate (VI) solution acidified with dilute sulfuric acid, propanone will be formed. Changing around with the reaction conditions makes no difference to the product.
We can see in the first reaction $A \to B$, oxidation of alcohol occurs in the presence of acidified potassium dichromate.
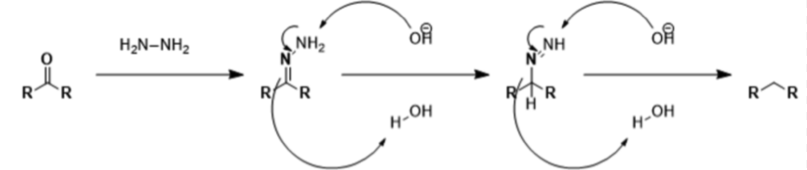
Wolff Kishner reduction mechanism
It begins with the formation of a hydrazone anion which further releases the nitrogen atom to form a carbanion. This carbanion further reacts with the water in the system to give a hydrocarbon. Typically, diethylene glycol is used as a solvent for the method.
This reduction is an organic reaction where the aldehydes and ketones are reduced to alkanes.
In the reaction $B \to C$, Wolf kishner reaction occurs in the presence of $N{H_2}N{H_2}$ and $KOH$ which makes B a ketone.
If B is a ketone that means, A has to be a second degree alcohol
Thus out of the options, option (D) is a second degree alcohol
So, correct answer is (D).
Note:
-Oxidation of alcohols is as follows:
${1^ \circ }$ alcohol $ \to $ Carboxylic acid
${2^ \circ }$ alcohol $ \to $ Ketone
${3^ \circ }$ alcohol $ \to $ No reaction
-If an alcohol is tertiary, it gives no result because it cannot be oxidized. If an alcohol is primary then it should be ethanol (as this is oxidized to ethanol, which is the only aldehyde which gives a positive result with the iodoform test)
-All secondary alcohols give a positive result since they are easily oxidized to ketones.
-Reaction $\,A \to B\,$ involves oxidation of secondary alcohols to ketones in the presence of acidified potassium dichromate.
-Reaction $\,B \to C\,$ involves the Wolff Kishner reduction mechanism where the ketone is converted to corresponding alkane.
-The reaction of B with aqueous $\,NaOH\,$ and iodine is the iodoform reaction which produces a yellow precipitate.
Complete step by step answer:
Iodoform reaction
All compounds containing the $C{H_3}C = O$ group or the $C{H_3}CH(OH)$ group give a positive result with the iodoform test. When iodine and sodium hydroxide are added to a compound containing one of these groups, a pale yellow precipitate of iodoform is further formed.
The iodoform test can be used to identify aldehydes and ketones.
The iodoform test can also be used to differentiate between the alcohols. All secondary alcohols give a positive result since they are easily oxidized to ketones.
Oxidation of Secondary alcohols
Secondary alcohols are oxidized to ketones. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate (VI) solution acidified with dilute sulfuric acid, propanone will be formed. Changing around with the reaction conditions makes no difference to the product.
We can see in the first reaction $A \to B$, oxidation of alcohol occurs in the presence of acidified potassium dichromate.

Wolff Kishner reduction mechanism
It begins with the formation of a hydrazone anion which further releases the nitrogen atom to form a carbanion. This carbanion further reacts with the water in the system to give a hydrocarbon. Typically, diethylene glycol is used as a solvent for the method.
This reduction is an organic reaction where the aldehydes and ketones are reduced to alkanes.
In the reaction $B \to C$, Wolf kishner reaction occurs in the presence of $N{H_2}N{H_2}$ and $KOH$ which makes B a ketone.
If B is a ketone that means, A has to be a second degree alcohol
Thus out of the options, option (D) is a second degree alcohol
So, correct answer is (D).
Note:
-Oxidation of alcohols is as follows:
${1^ \circ }$ alcohol $ \to $ Carboxylic acid
${2^ \circ }$ alcohol $ \to $ Ketone
${3^ \circ }$ alcohol $ \to $ No reaction
-If an alcohol is tertiary, it gives no result because it cannot be oxidized. If an alcohol is primary then it should be ethanol (as this is oxidized to ethanol, which is the only aldehyde which gives a positive result with the iodoform test)
-All secondary alcohols give a positive result since they are easily oxidized to ketones.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




