
Assertion:The downward displacement of air method used for drying and collecting ammonia gas
Reason:Ammonia gas is lighter than air and hence collected by the downward displacement of air. Ammonia is not collected over water since it is highly soluble in water (1 vol. of water dissolves about 702 vol. at and 1 atm pressure). The drying agent used is calcium oxide.
A.Both assertion and reason are correct and the reason is the correct explanation for assertion
B.Both assertion and reason are correct and the reason is not the correct explanation for assertion
C.Assertion is correct but the reason is incorrect.
D.Both assertion and reason are incorrect
Answer
600.3k+ views
Hint:
Some gases are soluble in water while some are not. This is because the reactivity of different gases with water either yields no results or results in the formation of newer compounds due to this interaction.
Complete step by step answer:
Before getting to the question, let us first understand how ammonia gas is produced. For the production of ammonia, a process called upward delivery is used. In this process, gases which are lighter than air are produced. Just like hydrogen gas, ammonia gas is also lighter than air. In the setup of production of ammonia, the solid reactants are placed in a horizontal test tube and are allowed to react. This tube is connected to a collection apparatus which is placed vertically and is attached using a glass syringe.
When the solid reactants are reacting, the gases formed are collected in the collection apparatus. The lighter gases stay at the top layer of the container, while heavier gases like air are collected on the lower end and are thereby released. Hence, this process is also known as the downward displacement of air.
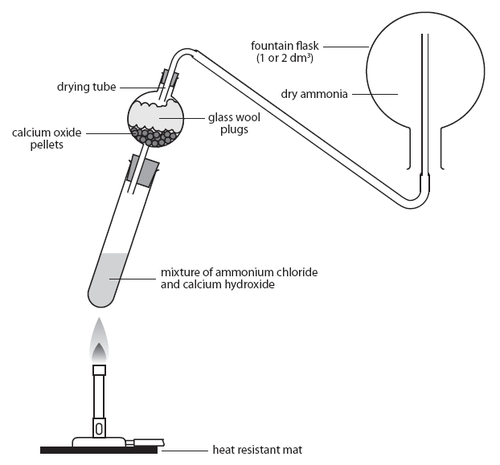
Also, ammonia is highly soluble in water. It reacts with water to form ammonium hydroxide. Hence, ammonia cannot be produced by downward displacement of water.
Hence, both assertion and reason are correct and the reason is not the correct explanation for assertion.
Hence, Option A is the correct option.
Note:
The collection method of different gases is decided upon their reactivity with the compounds involved in the different collection displacement methods. Three common methods to collect samples of gas are displacement of water, upwards delivery and downwards delivery.
Some gases are soluble in water while some are not. This is because the reactivity of different gases with water either yields no results or results in the formation of newer compounds due to this interaction.
Complete step by step answer:
Before getting to the question, let us first understand how ammonia gas is produced. For the production of ammonia, a process called upward delivery is used. In this process, gases which are lighter than air are produced. Just like hydrogen gas, ammonia gas is also lighter than air. In the setup of production of ammonia, the solid reactants are placed in a horizontal test tube and are allowed to react. This tube is connected to a collection apparatus which is placed vertically and is attached using a glass syringe.
When the solid reactants are reacting, the gases formed are collected in the collection apparatus. The lighter gases stay at the top layer of the container, while heavier gases like air are collected on the lower end and are thereby released. Hence, this process is also known as the downward displacement of air.

Also, ammonia is highly soluble in water. It reacts with water to form ammonium hydroxide. Hence, ammonia cannot be produced by downward displacement of water.
Hence, both assertion and reason are correct and the reason is not the correct explanation for assertion.
Hence, Option A is the correct option.
Note:
The collection method of different gases is decided upon their reactivity with the compounds involved in the different collection displacement methods. Three common methods to collect samples of gas are displacement of water, upwards delivery and downwards delivery.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




