
Arrange the following in the increasing order of acidity and justify the same.
\[{(C{H_3})_3}COH,\;\,C{H_3}OH,\;{(C{H_3})_2}CHOH\].
Answer
565.5k+ views
Hint:The acidic order of alcohol is determined by the stability of the conjugate base anion is checked. To find out the acidic order of the given alcohols we have to check the stability of their conjugate base anion.
Complete answer:
Alcohols are the organic compounds that have a $ - OH$ group. In this $ - OH$ bond, oxygen is more electronegative, and hence due to its more electronegative character, it shifts the electron cloud pair to its side and ${H^ + }$is released from the molecule. Hence acid shows an acidic character.
After losing the ${H^ + }$ alcohol will convert into alkoxide ion. The stability of these alkoxide ions decides the strength of the acidic character of the alcohol. More the stability of the alkoxide ion, more acidic will be the alcohol.
Now, we will check the stability of the primary, secondary, and tertiary alcohols, by comparing the stability of the alkoxide ions.
The stabilized alkoxide weaker will be a conjugate base and hence the alcohol will be more acidic. To calculate the strength of the alkoxide we will look at steric hindrance and electronic factors.
Alkoxide ion of primary, secondary, and tertiary alcohols will be:
$C{H_3} - {O^ - },\,$,
 and
and
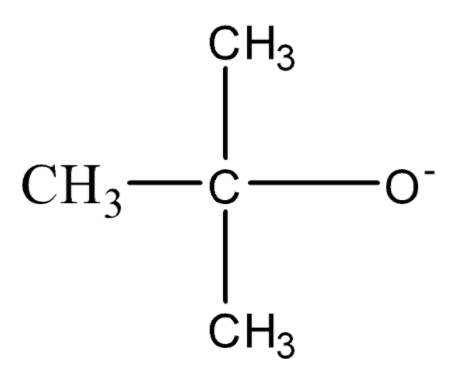
The presence of the methyl group induces an $ + I$ effect on the chain due to which the negative charge on the species will increase and the stability of the alkoxide ion will decrease and the strength of the conjugate base will increase.
More the number of $C{H_3}$ groups on-chain, less will be the stability of alkoxide ion and so will be the acidic character of alcohol.
Hence the correct order of acidity of alcohols will be;
${1^0} > {2^0} > {3^0}$
\[\;\,C{H_3}OH > \;{(C{H_3})_2}CHOH > {(C{H_3})_3}COH\]
Note:
In Alcohols primary alcohols are more acidic as compared to secondary alcohols which are more acidic as compared to tertiary alcohols. This is due to the weaker alkoxide ion which is due to the presence of $C{H_3}$ groups.
Complete answer:
Alcohols are the organic compounds that have a $ - OH$ group. In this $ - OH$ bond, oxygen is more electronegative, and hence due to its more electronegative character, it shifts the electron cloud pair to its side and ${H^ + }$is released from the molecule. Hence acid shows an acidic character.
After losing the ${H^ + }$ alcohol will convert into alkoxide ion. The stability of these alkoxide ions decides the strength of the acidic character of the alcohol. More the stability of the alkoxide ion, more acidic will be the alcohol.
Now, we will check the stability of the primary, secondary, and tertiary alcohols, by comparing the stability of the alkoxide ions.
The stabilized alkoxide weaker will be a conjugate base and hence the alcohol will be more acidic. To calculate the strength of the alkoxide we will look at steric hindrance and electronic factors.
Alkoxide ion of primary, secondary, and tertiary alcohols will be:
$C{H_3} - {O^ - },\,$,


The presence of the methyl group induces an $ + I$ effect on the chain due to which the negative charge on the species will increase and the stability of the alkoxide ion will decrease and the strength of the conjugate base will increase.
More the number of $C{H_3}$ groups on-chain, less will be the stability of alkoxide ion and so will be the acidic character of alcohol.
Hence the correct order of acidity of alcohols will be;
${1^0} > {2^0} > {3^0}$
\[\;\,C{H_3}OH > \;{(C{H_3})_2}CHOH > {(C{H_3})_3}COH\]
Note:
In Alcohols primary alcohols are more acidic as compared to secondary alcohols which are more acidic as compared to tertiary alcohols. This is due to the weaker alkoxide ion which is due to the presence of $C{H_3}$ groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




