
Arrange the following in decreasing order of electrophilicity.
I
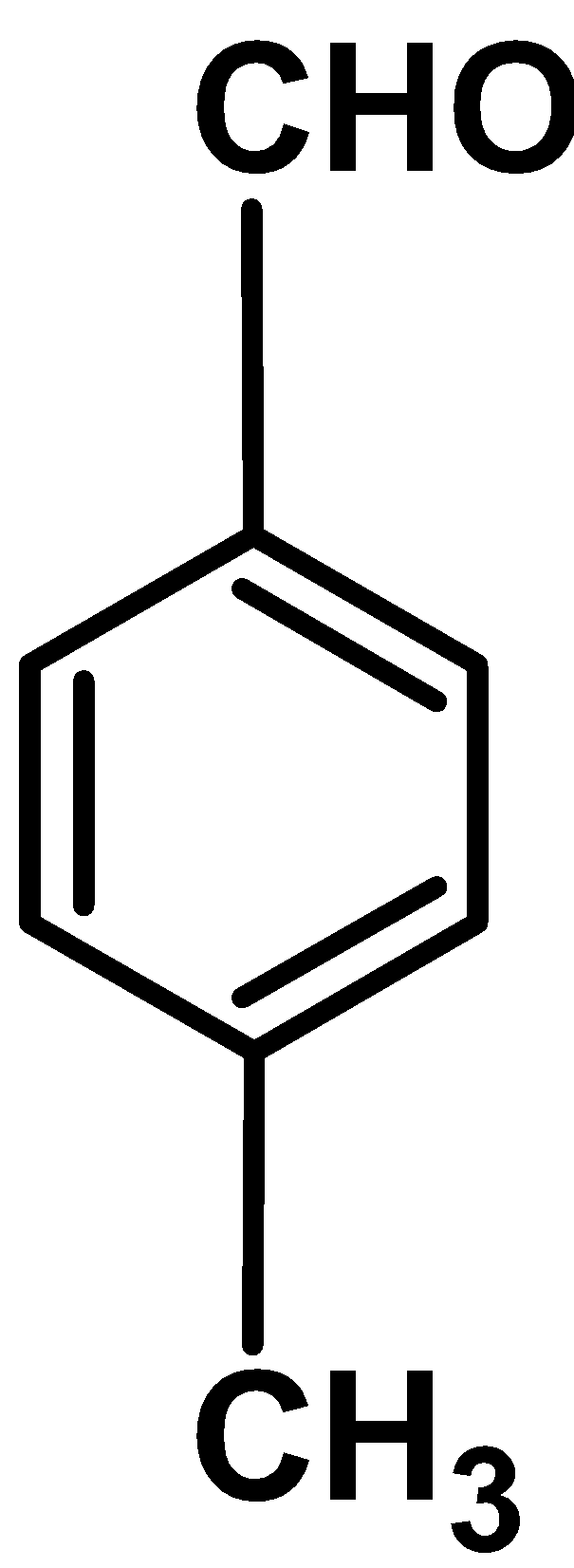
II
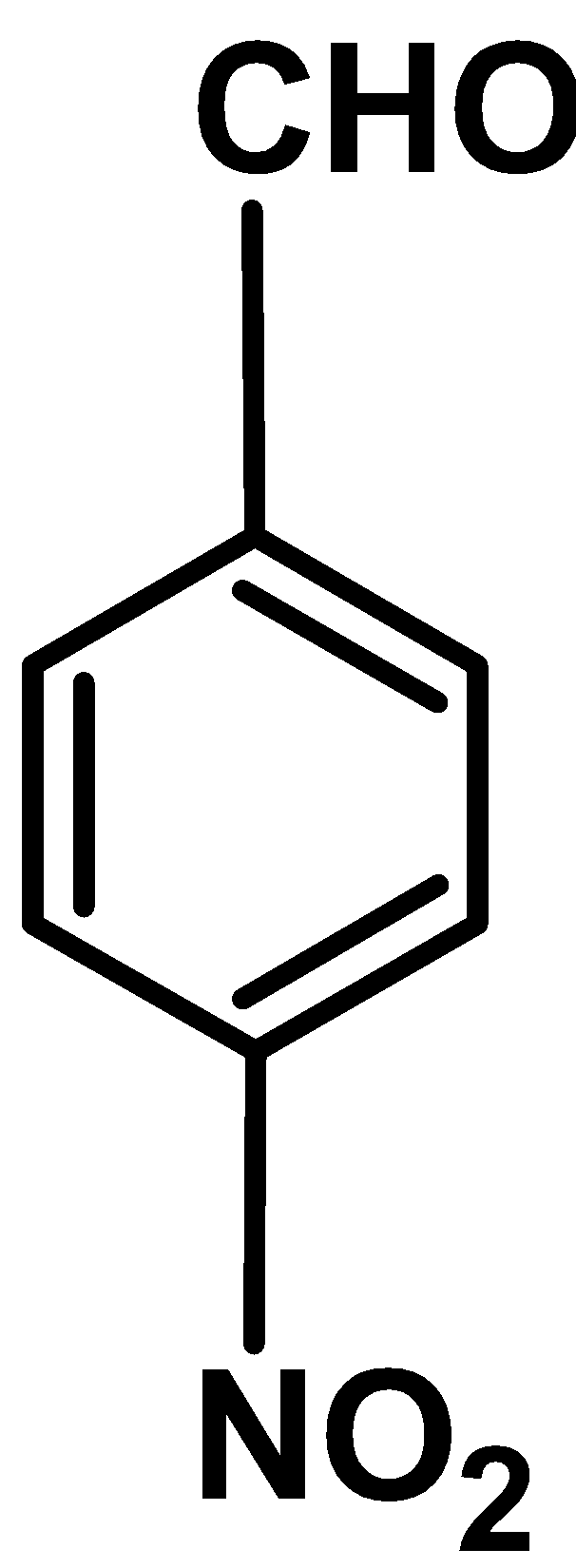
III
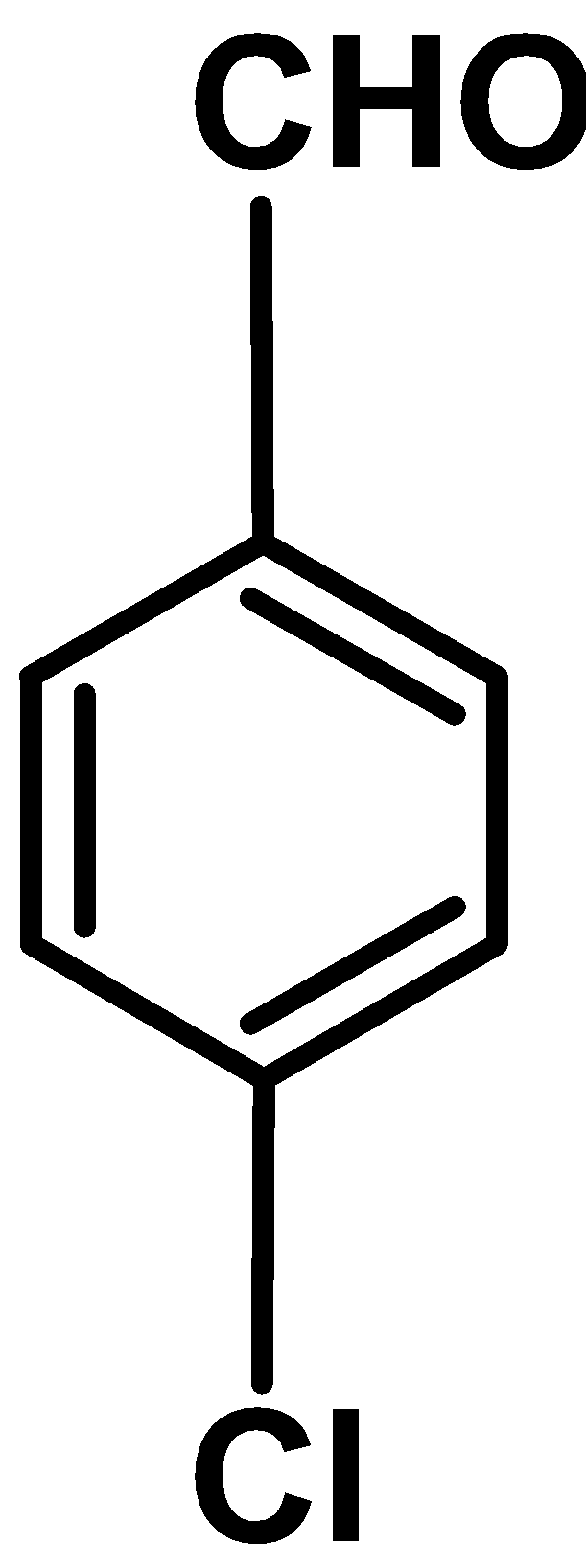
IV
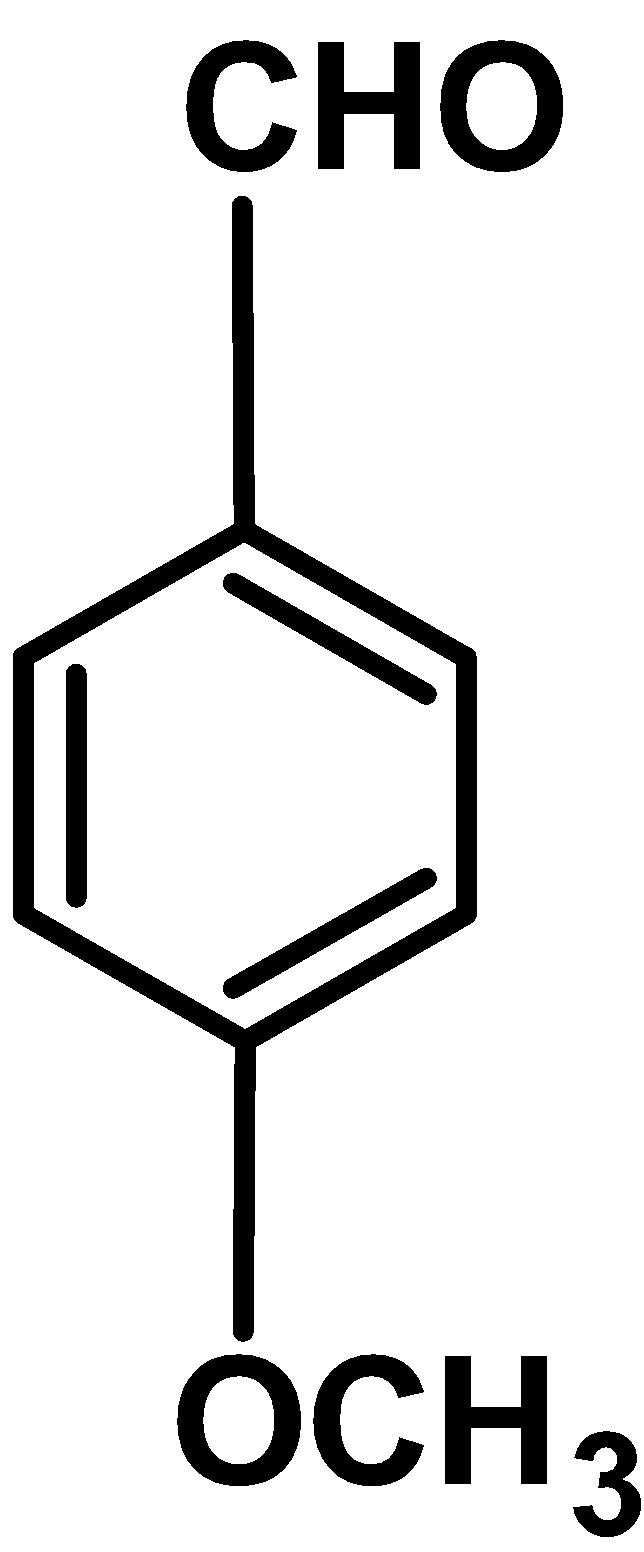
| I | 
|
| II | 
|
| III | 
|
| IV | 
|
Answer
573.9k+ views
Hint: Electrophile is an electron-deficient species .The extent to which the electrophile attacks on the molecule depends on the electrophilicity of the molecule. electrophility depends on the substituents on the benzene ring. The electron-withdrawing group reduces the electron density and deactivates the ring towards the electrophilic attack .The electron donating group activates the ring towards the ring.
Complete step by step solution:
In chemistry ,the electrophile is a species which is an electron deficient species and have a tendency to accept the electron.This is also known as the electron loving species.Higher is the electron density of the ring the more reactive towards the electrophilic attack.
The benzene ring is a rich source of electron.It has a higher electron density. Thus ,it is easily attacked by the electrophile. Since electrophiles are electron loving species , the benzene ring readily undergoes the electrophilic attack.
Electrophilicity of a ring depends on the nature of substituents on the ring.
Electron withdrawing group : Electron withdrawing group like $\text{ }-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ , $\text{ }-\text{Cl }$ wide was the electron density from the benzene ring towards itself.These reduces the electron density form the ring and thus ring deactivates towards the electrophilic
Electron donating group : Electron withdrawing group like \[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] , $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ releases the electron density to the benzene ring .These increase the electron density of the ring and thus ring is activated towards the electrophilic attack.
Out comparing methoxy\[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] and methyl $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$group , methoxy groups donates the electron density via resonance effect and methyl groups donates electron density via inductive effect $\text{ +I }$. Thus \[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] group activates the benzaldehyde more than attack.
Out comparing nitro $\text{ }-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ and chloro $\text{ }-\text{Cl }$ group ,nitro groups withdraws the electron density via resonance effect and chloro groups withdraws electron density via inductive effect $\text{ }-\text{I }$. Thus the nitro group deactivates the benzaldehyde to more extent than the chlorine substituted benzaldehyde.
the $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ substituted benzaldehyde.
Thus, the decreasing order of the electrophilicity of the substituted benzaldehyde is given as follows,
$\text{ II }<\text{ III }<\text{ I }<\text{ IV }$
Note: Note that , electron donating or ring activating group like methyl or methoxy group facilitates the electrophilic substitution at the ortho and para positions. These are called the ortho para directing groups.As this position has high electron density. However ,electron withdrawing groups like nitro or chloro group are meta directing groups ,as meta position is an electron deficient position.
Complete step by step solution:
In chemistry ,the electrophile is a species which is an electron deficient species and have a tendency to accept the electron.This is also known as the electron loving species.Higher is the electron density of the ring the more reactive towards the electrophilic attack.
The benzene ring is a rich source of electron.It has a higher electron density. Thus ,it is easily attacked by the electrophile. Since electrophiles are electron loving species , the benzene ring readily undergoes the electrophilic attack.
Electrophilicity of a ring depends on the nature of substituents on the ring.
Electron withdrawing group : Electron withdrawing group like $\text{ }-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ , $\text{ }-\text{Cl }$ wide was the electron density from the benzene ring towards itself.These reduces the electron density form the ring and thus ring deactivates towards the electrophilic
Electron donating group : Electron withdrawing group like \[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] , $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ releases the electron density to the benzene ring .These increase the electron density of the ring and thus ring is activated towards the electrophilic attack.
Out comparing methoxy\[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] and methyl $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$group , methoxy groups donates the electron density via resonance effect and methyl groups donates electron density via inductive effect $\text{ +I }$. Thus \[\text{ }-\text{OC}{{\text{H}}_{\text{3}}}\text{ }\] group activates the benzaldehyde more than attack.
Out comparing nitro $\text{ }-\text{N}{{\text{O}}_{\text{2}}}\text{ }$ and chloro $\text{ }-\text{Cl }$ group ,nitro groups withdraws the electron density via resonance effect and chloro groups withdraws electron density via inductive effect $\text{ }-\text{I }$. Thus the nitro group deactivates the benzaldehyde to more extent than the chlorine substituted benzaldehyde.
the $\text{ }-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ substituted benzaldehyde.
Thus, the decreasing order of the electrophilicity of the substituted benzaldehyde is given as follows,
$\text{ II }<\text{ III }<\text{ I }<\text{ IV }$
Note: Note that , electron donating or ring activating group like methyl or methoxy group facilitates the electrophilic substitution at the ortho and para positions. These are called the ortho para directing groups.As this position has high electron density. However ,electron withdrawing groups like nitro or chloro group are meta directing groups ,as meta position is an electron deficient position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




