
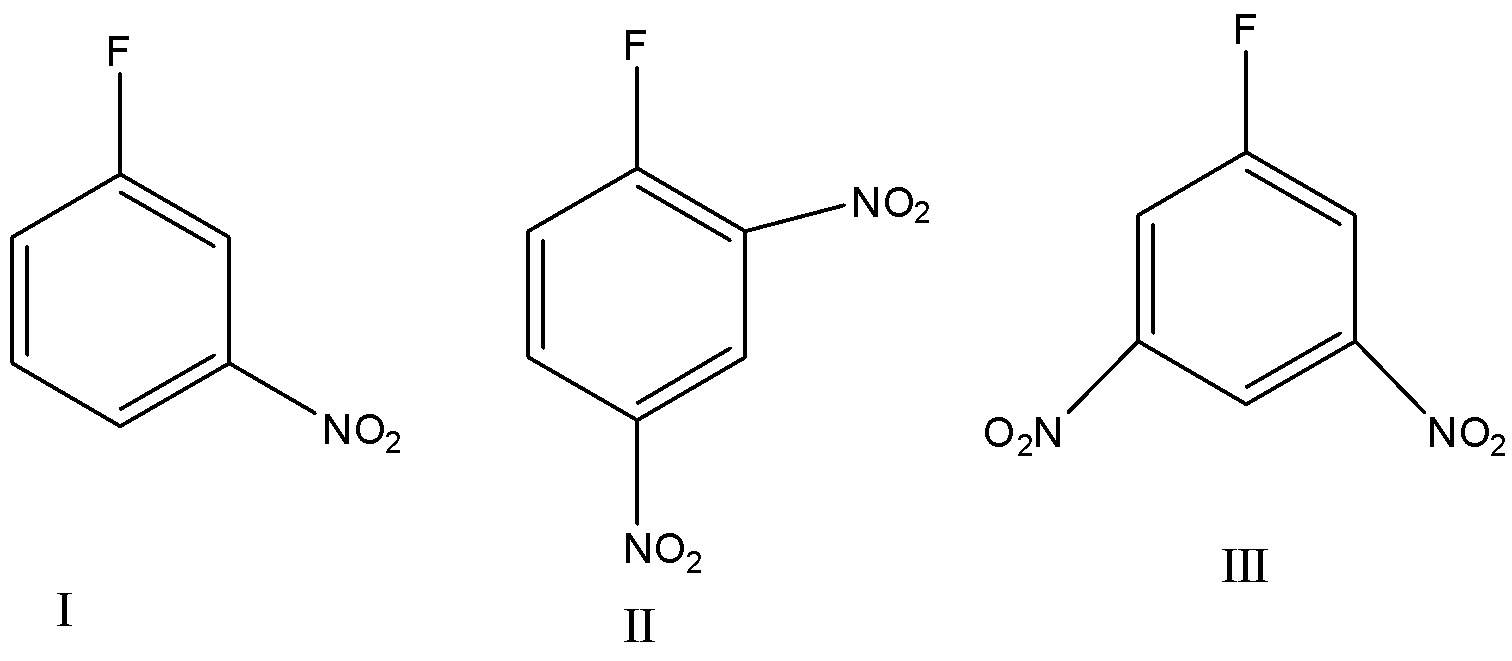
Arrange the above given compounds in increasing order of reactivity with $C{H_3}ONa$.
(A) $I < II < III$
(B) $III < II < I$
(C) $II < III < I$
(D) $I < III < II$

Answer
564.9k+ views
Hint: Reaction of given nitro compounds with sodium methoxide will result in a nucleophilic substitution reaction. So, in order to compare these compounds, we have to examine their reactivity in a nucleophilic substitution reaction.
Complete answer:
As we know both Nitro and Fluorine are electron withdrawing groups, so due to their electron-withdrawing nature, they will attract the electrons from the ring towards itself due to resonance which will result in the deficiency of electrons and the instability of the ring. The presence of $N{O_2}$ group at the ortho and para positions increases the reactivity of the Nucleophilic substitution reaction. Due to the decrease in electron density of the benzene ring the nucleophile can easily attack these sites. In sodium methoxide, the methoxide ion is the nucleophile and the fluorine atom is the leaving group. More the number of Nitro groups present in the ortho and para positions, more will be the reactivity. So, by this, we can conclude that $II$will be the most reactive compound followed by compound $III$. $I$ will be least reactive as it contains only one $N{O_2}$ group. Hence the order of reactivity will be: $I < III < II$.
Therefore option (D) is correct.
Additional Information:
Nucleophilic substitution reactions are those reactions in which a nucleophile that is rich in electrons attacks a positively charged electrophile and replaces the leaving group from the compound. It is a three-step process. It is generally of two types $S{N_{1\,}}\,$ and $S{N_2}$
Note:
The presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution because $N{O_2}$ group is an electron-withdrawing group that decreases the electron density of the benzene ring.
Complete answer:
As we know both Nitro and Fluorine are electron withdrawing groups, so due to their electron-withdrawing nature, they will attract the electrons from the ring towards itself due to resonance which will result in the deficiency of electrons and the instability of the ring. The presence of $N{O_2}$ group at the ortho and para positions increases the reactivity of the Nucleophilic substitution reaction. Due to the decrease in electron density of the benzene ring the nucleophile can easily attack these sites. In sodium methoxide, the methoxide ion is the nucleophile and the fluorine atom is the leaving group. More the number of Nitro groups present in the ortho and para positions, more will be the reactivity. So, by this, we can conclude that $II$will be the most reactive compound followed by compound $III$. $I$ will be least reactive as it contains only one $N{O_2}$ group. Hence the order of reactivity will be: $I < III < II$.
Therefore option (D) is correct.
Additional Information:
Nucleophilic substitution reactions are those reactions in which a nucleophile that is rich in electrons attacks a positively charged electrophile and replaces the leaving group from the compound. It is a three-step process. It is generally of two types $S{N_{1\,}}\,$ and $S{N_2}$
Note:
The presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution because $N{O_2}$ group is an electron-withdrawing group that decreases the electron density of the benzene ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




