
Why are the compounds of transition metal generally colored?
(A) Due to s being filled before d (Aufbau principle)
(B) Due to the presence of unpaired electron
(C) Unfilled d orbital
(D) d-d transition
Answer
583.2k+ views
Hint: The transition metal atoms belong to the d block elements. When a visible light falls on the compound or an ion, there is excitation of electrons from ground state to the excited state.
Complete step by step solution:
Most of the transition metals are colored both in solid as well as in the aqueous state. The transition metal or the d block elements are often colored due to the transition of electrons between the d orbitals of different energies. The transition metals also have unpaired electrons to form colored compounds.
So, when a visible light falls on the compound, the unpaired electron present in the lowest energy d orbital gets excited and promoted to the highest energy d-orbital. This is called the d-d transition of electrons. The d orbital splits into two sets when an anion or a ligand approach the transition metal. The splitting of d orbital is shown below:
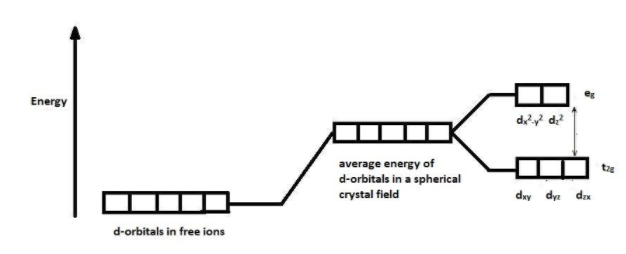
One set contains orbitals of low energy i.e.${{d}_{xy}},{{d}_{yz}}and\text{ }{{\text{d}}_{zx}}$ and the other set contains orbitals of high energy i.e.${{d}_{{{x}^{2}}-{{y}^{2}}}}\text{ and }{{\text{d}}_{{{z}^{2}}}}$. This is known as the crystal field splitting.
Hence the correct answer is option (B), (C) and (D) i.e. Due to the presence of unpaired electron, Unfilled d orbital, d-d transition.
Note: According to the Aufbau principle states that in the ground state of an atom or an ion, electrons fill the atomic orbitals of the lowest available energy level before occupying the highest energy levels. Due to this principle the electrons in an atom or an ion forms the most stable electronic configuration.
Complete step by step solution:
Most of the transition metals are colored both in solid as well as in the aqueous state. The transition metal or the d block elements are often colored due to the transition of electrons between the d orbitals of different energies. The transition metals also have unpaired electrons to form colored compounds.
So, when a visible light falls on the compound, the unpaired electron present in the lowest energy d orbital gets excited and promoted to the highest energy d-orbital. This is called the d-d transition of electrons. The d orbital splits into two sets when an anion or a ligand approach the transition metal. The splitting of d orbital is shown below:

One set contains orbitals of low energy i.e.${{d}_{xy}},{{d}_{yz}}and\text{ }{{\text{d}}_{zx}}$ and the other set contains orbitals of high energy i.e.${{d}_{{{x}^{2}}-{{y}^{2}}}}\text{ and }{{\text{d}}_{{{z}^{2}}}}$. This is known as the crystal field splitting.
Hence the correct answer is option (B), (C) and (D) i.e. Due to the presence of unpaired electron, Unfilled d orbital, d-d transition.
Note: According to the Aufbau principle states that in the ground state of an atom or an ion, electrons fill the atomic orbitals of the lowest available energy level before occupying the highest energy levels. Due to this principle the electrons in an atom or an ion forms the most stable electronic configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




