
Why are all the $P - F$ bonds pf $P{F_5}$ molecule not of the same bond length?
Answer
573.9k+ views
Hint:The bond length, also known as the bond distance is defined as the average distance between the nuclei of the two bonded atoms in a molecule. Generally, the shorter is the bond, more stronger is the bond and vice-versa.
Complete step by step answer:
For $P{F_5}$
Hybridization $ = \dfrac{1}{2}\left[ {V + M - C + A} \right]$
Where V= No. Of valence electrons of central atom
M- No. Of monovalent atom
C- Total Cation charge
A- Total Anion charge
$ = \dfrac{1}{2}\left[ {5 + 5 - 0 + 0} \right]$
$ = \dfrac{{10}}{2} = 5$
Hybridization is $s{p^3}d$.
$P{F_5}$ has three axial bonds and two equatorial bonds.
Axial bonds experience more repulsion due to bond angle of $90^\circ $ with $F$ - atom present at the equatorial position from both sides and it leads to long bonds. So axial bonds are longer than equatorial bonds.
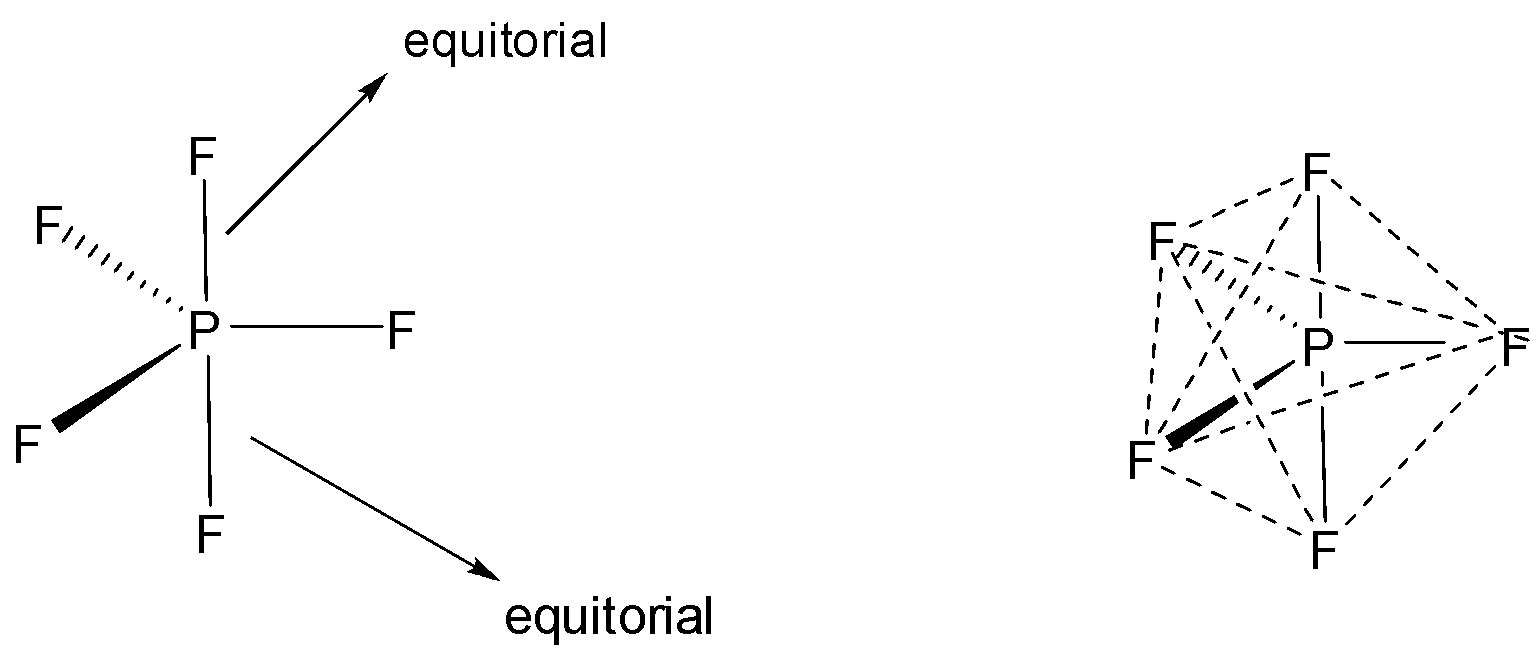
Axial $F$ atoms are in a different environment to equatorial $F$ atoms.
Summing up, we can say that $PF$ is $s{p^3}d$ hybridized and the shape of the molecule is trigonal bipyramidal. There atoms of $F$ will occupy equatorial position with bond angle of $120$degrees while other two $F$ atoms placed at equatorial position with bond angle of $180$ degrees.
The axial bonds are experiencing more repulsion ( due to bond angle of $90^\circ $ with $F$ atom present at equatorial position from both sides because they are perpendicular to each other ) than those of the equatorial and this leads to longer bonds. Bond length of the axial bond is greater than the equatorial bond. Therefore the bond will be different with respect to axial and equatorial position.
Additional Information: The bond length varies in accordance with the size of the atoms and the multiplicity of bonds. As the size of the atom increases, the bond length also gets increased while the bond length decreases with the increase in multiplicity of bonds (which is also known as bond order). Moreover, the oxidation number of the central atom and the percentage s-character of the orbital used to make the bond also affects the bond length.
Note:
The molecular geometry of $P{F_5}$ is trigonal bipyramidal with symmetric charge distribution. Therefore this molecule is nonpolar. Phosphorus pentafluoride is a colorless, poisonous, non-flammable, compressed gas with a pungent odour.
Complete step by step answer:
For $P{F_5}$
Hybridization $ = \dfrac{1}{2}\left[ {V + M - C + A} \right]$
Where V= No. Of valence electrons of central atom
M- No. Of monovalent atom
C- Total Cation charge
A- Total Anion charge
$ = \dfrac{1}{2}\left[ {5 + 5 - 0 + 0} \right]$
$ = \dfrac{{10}}{2} = 5$
Hybridization is $s{p^3}d$.
$P{F_5}$ has three axial bonds and two equatorial bonds.
Axial bonds experience more repulsion due to bond angle of $90^\circ $ with $F$ - atom present at the equatorial position from both sides and it leads to long bonds. So axial bonds are longer than equatorial bonds.

Axial $F$ atoms are in a different environment to equatorial $F$ atoms.
Summing up, we can say that $PF$ is $s{p^3}d$ hybridized and the shape of the molecule is trigonal bipyramidal. There atoms of $F$ will occupy equatorial position with bond angle of $120$degrees while other two $F$ atoms placed at equatorial position with bond angle of $180$ degrees.
The axial bonds are experiencing more repulsion ( due to bond angle of $90^\circ $ with $F$ atom present at equatorial position from both sides because they are perpendicular to each other ) than those of the equatorial and this leads to longer bonds. Bond length of the axial bond is greater than the equatorial bond. Therefore the bond will be different with respect to axial and equatorial position.
Additional Information: The bond length varies in accordance with the size of the atoms and the multiplicity of bonds. As the size of the atom increases, the bond length also gets increased while the bond length decreases with the increase in multiplicity of bonds (which is also known as bond order). Moreover, the oxidation number of the central atom and the percentage s-character of the orbital used to make the bond also affects the bond length.
Note:
The molecular geometry of $P{F_5}$ is trigonal bipyramidal with symmetric charge distribution. Therefore this molecule is nonpolar. Phosphorus pentafluoride is a colorless, poisonous, non-flammable, compressed gas with a pungent odour.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




