
Aniline reacts with which of these to form Schiff’s base?
A. Acetic acid
B. Benzaldehyde
C. Acetone
D. \[N{H_3}\]
Answer
233.1k+ views
Hint: Schiff’s base is an organic compound containing \[ - C = N - \], an imine bond. It is produced by the reaction between aromatic or aliphatic amines with a compound containing an aldehyde group.
Complete Step by Step Solution:
In this reaction to form Schiff’s base, firstly nucleophilic addition and then dehydration takes place with the elimination of water. Hence, it is a condensation reaction. As we know the structure of Schiff’s base, so, by comparing the structure of Schiff’s base and aniline, we can find out that benzaldehyde is the one which will react with aniline to give the structure same as Schiff’s base. Benzaldehyde has an aldehyde group and will form Schiff base on a reaction with aniline in an acidic condition.
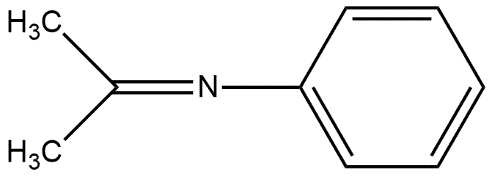
Image: Structure of Schiff’s base
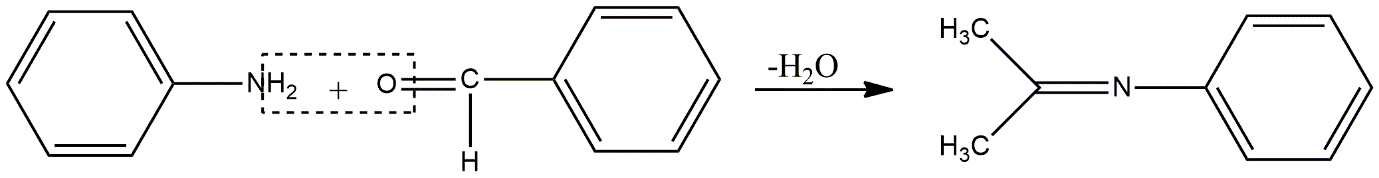
Image:Condensation reaction of aniline and benzaldehyde to form Schiff’s base
Here, hydrogens from aniline and oxygen from benzaldehyde will be eliminated in the form of neutral water to give Schiff’s base and this reaction takes place in acidic conditions where acid acts as a catalyst.
So, option B is correct.
Additional Information: Schiff base is used to distinguish between aldehyde and ketone as it gives reaction selectively with an aldehyde. Schiff bases are used in many reactions as a catalyst. They are also important for reactions in living organisms like decarboxylation and cleavage of carbon-carbon single bonds. These reactions are very necessary for the living system. From the options, acetic acid is an acid. It will not form Schiff’s base; it can help in a reaction as a catalyst.
Note: We use Schiff base mostly for those complexes which are in reaction to forming coordination complexes. Reaction to form Schiff’s base is a selective reaction for aldehyde..
Complete Step by Step Solution:
In this reaction to form Schiff’s base, firstly nucleophilic addition and then dehydration takes place with the elimination of water. Hence, it is a condensation reaction. As we know the structure of Schiff’s base, so, by comparing the structure of Schiff’s base and aniline, we can find out that benzaldehyde is the one which will react with aniline to give the structure same as Schiff’s base. Benzaldehyde has an aldehyde group and will form Schiff base on a reaction with aniline in an acidic condition.

Image: Structure of Schiff’s base

Image:Condensation reaction of aniline and benzaldehyde to form Schiff’s base
Here, hydrogens from aniline and oxygen from benzaldehyde will be eliminated in the form of neutral water to give Schiff’s base and this reaction takes place in acidic conditions where acid acts as a catalyst.
So, option B is correct.
Additional Information: Schiff base is used to distinguish between aldehyde and ketone as it gives reaction selectively with an aldehyde. Schiff bases are used in many reactions as a catalyst. They are also important for reactions in living organisms like decarboxylation and cleavage of carbon-carbon single bonds. These reactions are very necessary for the living system. From the options, acetic acid is an acid. It will not form Schiff’s base; it can help in a reaction as a catalyst.
Note: We use Schiff base mostly for those complexes which are in reaction to forming coordination complexes. Reaction to form Schiff’s base is a selective reaction for aldehyde..
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




