
An example of a sigma bonded organometallic compound is:
A) Cobaltocene
B) Ruthenocene
C) Grignard's reagent
D) Ferrocene
Answer
531.5k+ views
Hint: The organometallic compounds in which the metal ‘M’ and carbon of the ligand contains the covalent sigma bond are known as the sigma organometallic compounds.
$\text{ }\overset{\text{ }\!\!\delta\!\!\text{ }+}{\mathop{\text{M}}}\,-\overset{\text{ }\!\!\delta\!\!\text{ }-}{\mathop{\text{C}}}\,\text{ }$
The metal may be bonded to the alkyl, aryl, or the pi-bonded ligands such as cyclopentadienyl anion $\text{ (}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}^{-}}\text{ }$.
Complete step by step answer:
Organometallic are those compounds which contain one or more metal-carbon bonds.
The organometallic compounds are classified based on the nature of the metal-carbon bond. Their classification is as follows:
1) $\text{ }\sigma -$ Bonded organometallics
2) $\text{ }\pi -$ Bonded organometallics
The compounds containing metal-carbon covalent sigma bonds are called as the $\text{ }\sigma -$ bonded organometallic compounds. One of the very popular examples of $\text{ }\sigma -$ bonded organometallics compounds is Grignard reagents. The Grignard reagent is an organometallic compound of magnesium. It has a general structure as $\text{ R}-\text{Mg}-\text{X }$ , where X is the halogen, R is an organic group. It can be alkyl or aryl.
Some of the common examples of Grignard reagent are as methyl magnesium chloride $\text{ Cl}-\text{Mg}-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and phenyl magnesium bromide$\text{ (}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{)}-\text{Mg}-\text{Br}$.
Here, we observe that the metal that is magnesium and the R group (aryl or alkyl) forms a covalent sigma bond .It is prepared by treating the alkyl or aryl halides with the magnesium metal in presence of dry ether. The Grignard reagent is very popular in organic synthesis, it creates a new carbon-carbon bond. Let's have a look at the other option.
The ferrocene is as shown below,
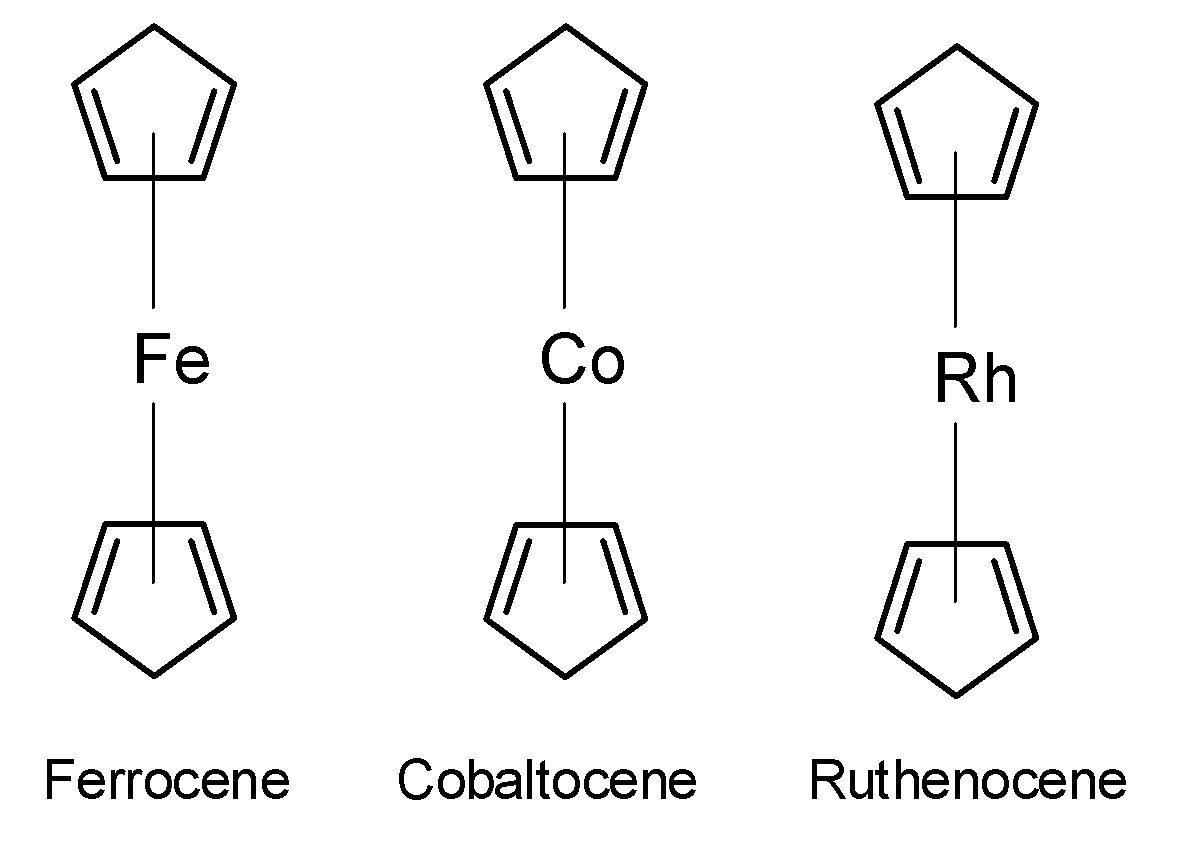
The ferrocene compound contains the two$\text{ Fe }-({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{) }$bonds. This $\text{ Fe }$ is sandwiched between the two cyclopentadienyl ligands. This is a pi bonded system. The plane of the rings is the same distance from the iron atom. Here, the $\text{ Fe }$ is bonded to the pi bond system thus the ferrocene is $\text{ }\pi -$ bonded organometallic compound.
The cobalt Cobaltocene $\text{ Co(}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}\text{ }$ and Ruthenocene $\text{ Rh(}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}\text{ }$have a similar structure to the ferrocene. So, Cobaltocene and Ruthenocene are also $\text{ }\pi -$ bonded organometallic compounds. Thus, the Grignard reagent is a sigma bonded organometallic compound. So, the correct answer is “Option C”.
Note: Some of the examples of the sigma bonded organometallic compounds are as follows:
1) Diethyl Zinc, $\text{ (}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{)Zn }$dimethyl magnesium, dimethyl beryllium, etc.
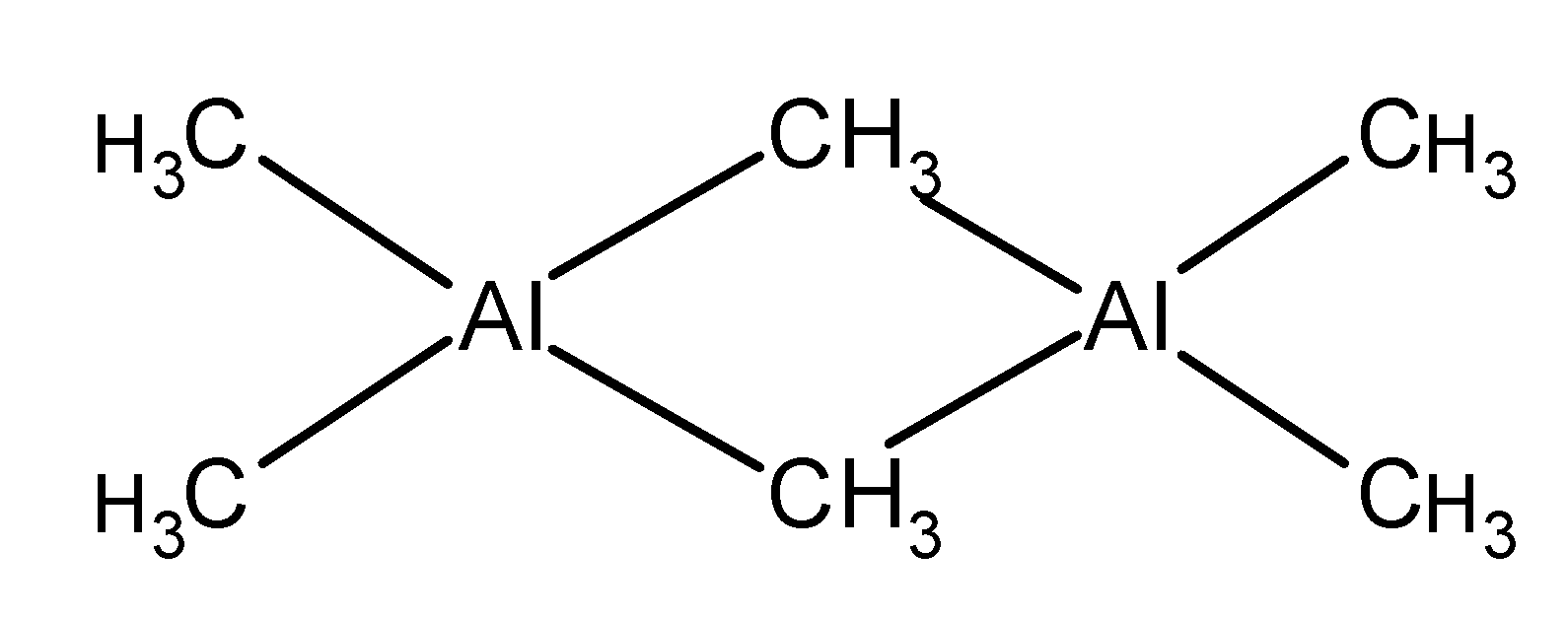
2) Trimethylaluminum$\text{ (C}{{\text{H}}_{\text{3}}}\text{)Al }$ . It exists as a dimer $\text{ }\left[ \text{A}{{\text{l}}_{\text{2}}}{{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{6}}} \right]\text{ }$ with two methyl groups acting as a bridge between two aluminium.
3) Tetraethyl tin, $\text{ Sn(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{4}}}\text{ }$ and tetra methyl tin $\text{ Sn(C}{{\text{H}}_{3}}{{\text{)}}_{\text{4}}}\text{ }$.
$\text{ }\overset{\text{ }\!\!\delta\!\!\text{ }+}{\mathop{\text{M}}}\,-\overset{\text{ }\!\!\delta\!\!\text{ }-}{\mathop{\text{C}}}\,\text{ }$
The metal may be bonded to the alkyl, aryl, or the pi-bonded ligands such as cyclopentadienyl anion $\text{ (}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}^{-}}\text{ }$.
Complete step by step answer:
Organometallic are those compounds which contain one or more metal-carbon bonds.
The organometallic compounds are classified based on the nature of the metal-carbon bond. Their classification is as follows:
1) $\text{ }\sigma -$ Bonded organometallics
2) $\text{ }\pi -$ Bonded organometallics
The compounds containing metal-carbon covalent sigma bonds are called as the $\text{ }\sigma -$ bonded organometallic compounds. One of the very popular examples of $\text{ }\sigma -$ bonded organometallics compounds is Grignard reagents. The Grignard reagent is an organometallic compound of magnesium. It has a general structure as $\text{ R}-\text{Mg}-\text{X }$ , where X is the halogen, R is an organic group. It can be alkyl or aryl.
Some of the common examples of Grignard reagent are as methyl magnesium chloride $\text{ Cl}-\text{Mg}-\text{C}{{\text{H}}_{\text{3}}}\text{ }$ and phenyl magnesium bromide$\text{ (}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{)}-\text{Mg}-\text{Br}$.
Here, we observe that the metal that is magnesium and the R group (aryl or alkyl) forms a covalent sigma bond .It is prepared by treating the alkyl or aryl halides with the magnesium metal in presence of dry ether. The Grignard reagent is very popular in organic synthesis, it creates a new carbon-carbon bond. Let's have a look at the other option.
The ferrocene is as shown below,

The ferrocene compound contains the two$\text{ Fe }-({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{) }$bonds. This $\text{ Fe }$ is sandwiched between the two cyclopentadienyl ligands. This is a pi bonded system. The plane of the rings is the same distance from the iron atom. Here, the $\text{ Fe }$ is bonded to the pi bond system thus the ferrocene is $\text{ }\pi -$ bonded organometallic compound.
The cobalt Cobaltocene $\text{ Co(}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}\text{ }$ and Ruthenocene $\text{ Rh(}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}\text{ }$have a similar structure to the ferrocene. So, Cobaltocene and Ruthenocene are also $\text{ }\pi -$ bonded organometallic compounds. Thus, the Grignard reagent is a sigma bonded organometallic compound. So, the correct answer is “Option C”.
Note: Some of the examples of the sigma bonded organometallic compounds are as follows:
1) Diethyl Zinc, $\text{ (}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{)Zn }$dimethyl magnesium, dimethyl beryllium, etc.
2) Trimethylaluminum$\text{ (C}{{\text{H}}_{\text{3}}}\text{)Al }$ . It exists as a dimer $\text{ }\left[ \text{A}{{\text{l}}_{\text{2}}}{{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{6}}} \right]\text{ }$ with two methyl groups acting as a bridge between two aluminium.

3) Tetraethyl tin, $\text{ Sn(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{4}}}\text{ }$ and tetra methyl tin $\text{ Sn(C}{{\text{H}}_{3}}{{\text{)}}_{\text{4}}}\text{ }$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




