
Among the following select the alkane that is expected to have lowest boiling point
A.Hexane
B.2-methylpentane
C.3-methylpentane
D.2,2-dimethylbutane
Answer
573.6k+ views
Hint:Boiling point is affected by the intermolecular forces that operate within a molecule. Mainly there are three types of molecular forces present- Hydrogen forces, London forces and dipole-dipole interactions.
Complete step by step answer:
-The temperature at which vapor pressure of liquid becomes equal to the atmospheric pressure is called the boiling point of the liquid
-Boiling points of hydrocarbons like alkanes are affected by several factors.
-For alkanes, the boiling point increases as molecular mass increases. This is because the intermolecular Van der Waals force increases as molecular size increases.
-Hexane, 2-methylpentane, 3-methylpentane and 2,2-dimethylbutane have the same molecular formula but different structural formula. Such compounds are called structural isomers of each other. These structures differ in the length of continuous parent carbon chains. The structure are as follows-
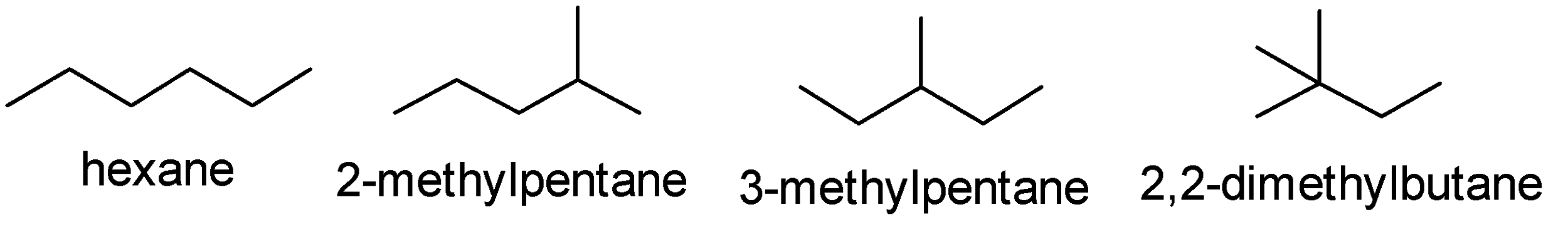
-For isomeric alkanes, we observe an interesting pattern in the boiling point. In case of isomeric alkanes the boiling point decreases as branching in the molecule increases. This is because as the number of branched chains in the molecule increases it gains the shape like a sphere. Now because of the spherical shape the molecule has less area of contact and therefore the intermolecular force between the spherical molecules becomes weak. Now these weak intermolecular forces can be overcome at relatively low temperatures.
-In the above structures we can see that 2,2-dimethylbutane has the highest branching as compared to the other structures. Hence it will have the lowest boiling point.
So the correct option is (D) .
Note:
-Boiling point increases as molecular mass increases. In case of isomers, boiling point decreases as branching in the molecule increases.
-The structures given above are isomers of alkane corresponding to the molecular formula \[{C_6}{H_{14}}\] .
-Boiling point indicates the physical state of the substance (liquid or gas) and also about the volatility of a compound.
Complete step by step answer:
-The temperature at which vapor pressure of liquid becomes equal to the atmospheric pressure is called the boiling point of the liquid
-Boiling points of hydrocarbons like alkanes are affected by several factors.
-For alkanes, the boiling point increases as molecular mass increases. This is because the intermolecular Van der Waals force increases as molecular size increases.
-Hexane, 2-methylpentane, 3-methylpentane and 2,2-dimethylbutane have the same molecular formula but different structural formula. Such compounds are called structural isomers of each other. These structures differ in the length of continuous parent carbon chains. The structure are as follows-

-For isomeric alkanes, we observe an interesting pattern in the boiling point. In case of isomeric alkanes the boiling point decreases as branching in the molecule increases. This is because as the number of branched chains in the molecule increases it gains the shape like a sphere. Now because of the spherical shape the molecule has less area of contact and therefore the intermolecular force between the spherical molecules becomes weak. Now these weak intermolecular forces can be overcome at relatively low temperatures.
-In the above structures we can see that 2,2-dimethylbutane has the highest branching as compared to the other structures. Hence it will have the lowest boiling point.
So the correct option is (D) .
Note:
-Boiling point increases as molecular mass increases. In case of isomers, boiling point decreases as branching in the molecule increases.
-The structures given above are isomers of alkane corresponding to the molecular formula \[{C_6}{H_{14}}\] .
-Boiling point indicates the physical state of the substance (liquid or gas) and also about the volatility of a compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




