
How is ammonia manufactured by Haber’s process? Explain
Answer
233.1k+ views
Hint: We should know that Haber’s process is an industrial process for the synthesis of ammonia. In this process, a particular catalyst is required at a particular temperature and pressure. Now you can try to explain this accordingly.
Complete step by step answer:
As we already discussed on an industrial scale, ammonia is prepared by Haber’s process.
The constituents of ammonia $N_{ 2 }$ and $H_{ 2 }$ combine in a ratio of 1:3.
$N_{ 2 }\quad +\quad 3H_{ 2 }\quad \rightleftharpoons \quad 2NH_{ 3 }$
The reaction proceeds in the forward direction with a remarkable decrease in volume. This reaction is exothermic.
In accordance with Le Chatelier’s principle, high pressure would favor the formation of ammonia.
The optimum conditions for the production of ammonia are a pressure of approx 200atm, a temperature of approx 700K, and the use of a catalyst such as iron with small amounts of $K_{ 2 }O$ and $Al_{ 2 }O_{ 3 }$ to increase the rate of attainment of equilibrium.
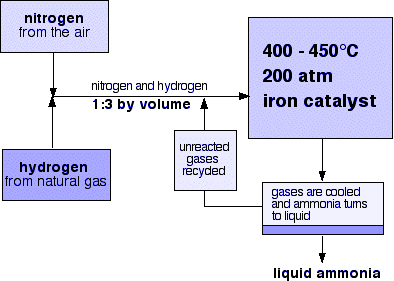
- As you can see in the diagram, in the Haber process, we take nitrogen gas from the air and combine it with a hydrogen atom obtained from natural gas in the ratio 1:3 by volume.
- Then these gases are passed through the catalyst, with cooling taking place in each pass. This is done to maintain equilibrium constant. Cooling is necessary because this reaction is exothermic.
- While different levels of conversion occur in each pass where unreacted gases are recycled.
- Normally, an iron catalyst is used in the process, and the whole procedure is conducted by maintaining a temperature of around 400 – 450$^{ 0 }C$ and a pressure of 150 – 200 atm.
- In the final stage of the process, the ammonia gas is cooled down to form a liquid solution which is then collected and stored in storage containers.
Therefore, we discussed the process of ammonia synthesis by Haber’s process.
Note: We should know that today, the Haber process is necessary because it produces ammonia, which is vital for fertilizer and many other purposes.
Every year, the Haber cycle produces around 500 million tons of fertilizer. This fertilizer helps feed about 40% of the population of the world.
Complete step by step answer:
As we already discussed on an industrial scale, ammonia is prepared by Haber’s process.
The constituents of ammonia $N_{ 2 }$ and $H_{ 2 }$ combine in a ratio of 1:3.
$N_{ 2 }\quad +\quad 3H_{ 2 }\quad \rightleftharpoons \quad 2NH_{ 3 }$
The reaction proceeds in the forward direction with a remarkable decrease in volume. This reaction is exothermic.
In accordance with Le Chatelier’s principle, high pressure would favor the formation of ammonia.
The optimum conditions for the production of ammonia are a pressure of approx 200atm, a temperature of approx 700K, and the use of a catalyst such as iron with small amounts of $K_{ 2 }O$ and $Al_{ 2 }O_{ 3 }$ to increase the rate of attainment of equilibrium.
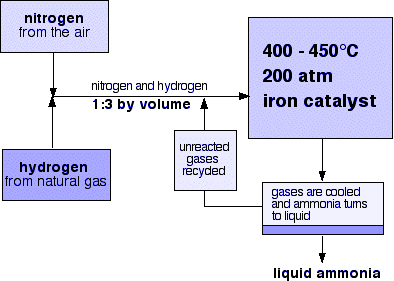
- As you can see in the diagram, in the Haber process, we take nitrogen gas from the air and combine it with a hydrogen atom obtained from natural gas in the ratio 1:3 by volume.
- Then these gases are passed through the catalyst, with cooling taking place in each pass. This is done to maintain equilibrium constant. Cooling is necessary because this reaction is exothermic.
- While different levels of conversion occur in each pass where unreacted gases are recycled.
- Normally, an iron catalyst is used in the process, and the whole procedure is conducted by maintaining a temperature of around 400 – 450$^{ 0 }C$ and a pressure of 150 – 200 atm.
- In the final stage of the process, the ammonia gas is cooled down to form a liquid solution which is then collected and stored in storage containers.
Therefore, we discussed the process of ammonia synthesis by Haber’s process.
Note: We should know that today, the Haber process is necessary because it produces ammonia, which is vital for fertilizer and many other purposes.
Every year, the Haber cycle produces around 500 million tons of fertilizer. This fertilizer helps feed about 40% of the population of the world.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




