
a)Describe the conformations of cyclohexanol. Comment on their stability.
b)How are the following conversions carried out?
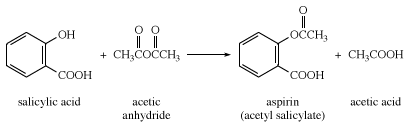
i.salicylic acid $\, \to \,$ aspirin
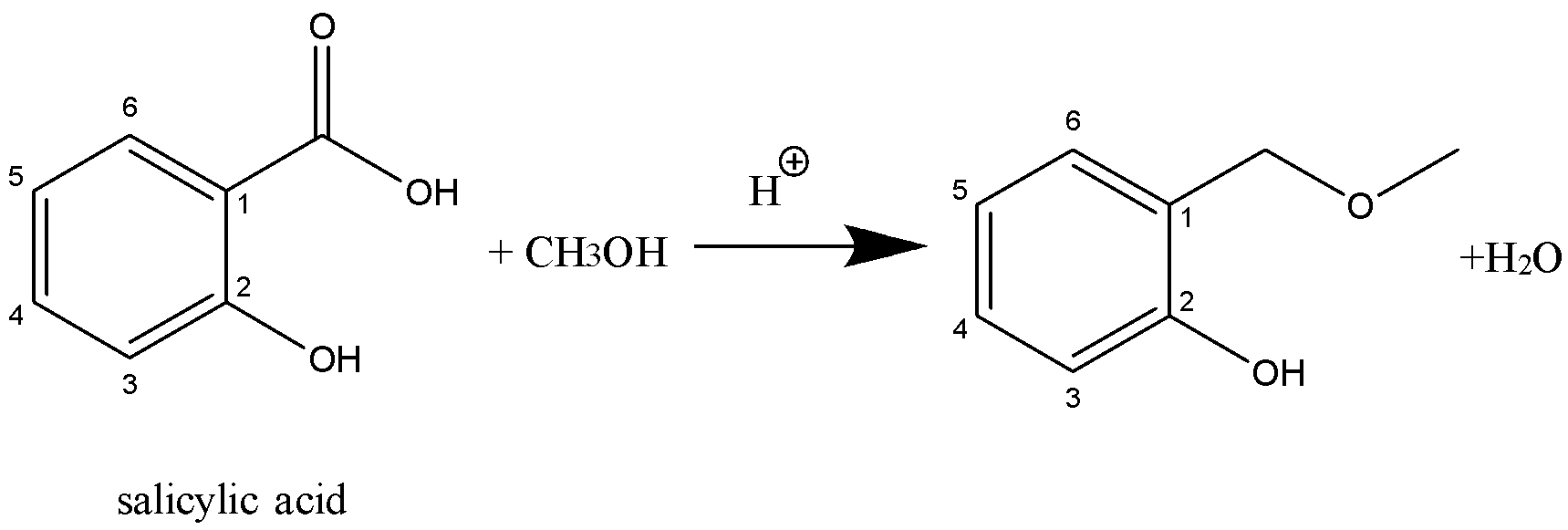
ii.salicylic acid $ \to $ methyl salicylate
iii.Formic acid $ \to $ formamide
Answer
573.6k+ views
Hint:The cyclohexane has an inclination to require up multiple warped conformations so the bond angles are brought nearer to the tetrahedral angle and there's reduced overall strain energy. The most common examples of conformations of cyclohexane are the boat, the twist-boat, the chair, and the half-chair conformations.
Complete step by step solution:
-A regular hexagon structure consists of internal angles of ${120^ \circ }$ . However, the carbon-carbon ($\,C - C\,$) bonds belonging to the cyclohexane ring have a tetrahedral symmetry, with the bond angles equivalent to ${109.5^ \circ }$ .
The chair form is the most stable conformation of cyclohexanes. The $\,C - C - C\,$ bonds are approximately ${109.5^ \circ }$ , which makes them nearly free from angle pressure. It is also a completely staggered conformation and It is free from torsional stress.

Boat conformation is the least stable and has the highest strength of all. It has torsional stress since each bond almost fully ellipses other bonds in the Newman projections.
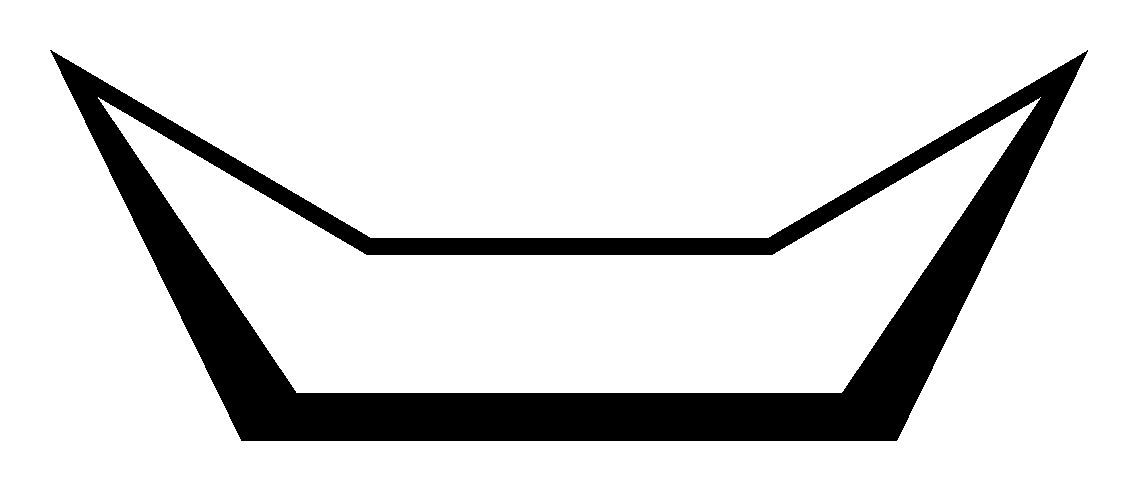
Twist boat conformation- The conformation is produced by twisting the boat to grant the twist or skew-boat conformation. The twist relieves little bit of the torsional strain of the boat and moves the flagpole H further apart thereby reducing the steric strain. Therefore, the twist boat is slightly more stable than the boat.
b) Conversions-
i.salicylic acid to aspirin conversion is carried forward as seen in the diagram below
ii.Methyl salicylate is obtained from the reaction between salicylic acid and methanol, also known as the oil of wintergreen.
Formic acid to formamide
iii.Formamide is produced by treating formic acid with ammonia, which produces ammonium formate which in turn yields formamide upon heating
$HCOOH + N{H_3} \to HCO{O^ - }N{H_4}^ + $
$HCO{O^ - }N{H_4}^ + \to HCON{H_2} + {H_2}O$
Note:
-The conformational rotation of cyclohexane transforms the conformations. This proceeds from one chair to twist boat to boat to twist boat to the opposite chair conformation. This process is understood as ring flipping.
-In the chair cyclohexane conformation there are two styles of positions, known as axial and equatorial. The axial positions are directed during a perpendicular position to the plane of the ring, and also the equatorial positions are round the plane of the ring. The adjacent axial positions point in opposite directions. The same is true for equatorial positions.
Complete step by step solution:
-A regular hexagon structure consists of internal angles of ${120^ \circ }$ . However, the carbon-carbon ($\,C - C\,$) bonds belonging to the cyclohexane ring have a tetrahedral symmetry, with the bond angles equivalent to ${109.5^ \circ }$ .
The chair form is the most stable conformation of cyclohexanes. The $\,C - C - C\,$ bonds are approximately ${109.5^ \circ }$ , which makes them nearly free from angle pressure. It is also a completely staggered conformation and It is free from torsional stress.

Boat conformation is the least stable and has the highest strength of all. It has torsional stress since each bond almost fully ellipses other bonds in the Newman projections.

Twist boat conformation- The conformation is produced by twisting the boat to grant the twist or skew-boat conformation. The twist relieves little bit of the torsional strain of the boat and moves the flagpole H further apart thereby reducing the steric strain. Therefore, the twist boat is slightly more stable than the boat.

b) Conversions-
i.salicylic acid to aspirin conversion is carried forward as seen in the diagram below

ii.Methyl salicylate is obtained from the reaction between salicylic acid and methanol, also known as the oil of wintergreen.

Formic acid to formamide
iii.Formamide is produced by treating formic acid with ammonia, which produces ammonium formate which in turn yields formamide upon heating
$HCOOH + N{H_3} \to HCO{O^ - }N{H_4}^ + $
$HCO{O^ - }N{H_4}^ + \to HCON{H_2} + {H_2}O$
Note:
-The conformational rotation of cyclohexane transforms the conformations. This proceeds from one chair to twist boat to boat to twist boat to the opposite chair conformation. This process is understood as ring flipping.
-In the chair cyclohexane conformation there are two styles of positions, known as axial and equatorial. The axial positions are directed during a perpendicular position to the plane of the ring, and also the equatorial positions are round the plane of the ring. The adjacent axial positions point in opposite directions. The same is true for equatorial positions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




