
Acetylation of a polyhydric alcohol increases its molecular mass by $126$ units. The number of ‘$OH$’ groups in the molecule is:
A. $2$
B. $3$
C. $4$
D. $5$
Answer
573.6k+ views
Hint: During acetylation, the acetyl group substitutes the hydrogen atoms present in the ‘$OH$’ groups of the molecule. Thus, the change in mass is actually the difference in molecular mass of an acetyl group and hydrogen. Subtracting the molar mass of the acetyl group and the atomic mass of hydrogen will thus give us the increase in mass caused by one acetyl group. Dividing the total change in mass by the change in mass caused by one acetyl group will give us the number of ‘$OH$’ groups in the molecule.
Complete step by step solution:
During acetylation of an alcohol, the acetyl group ($C{H_3}(CO)$) substitutes the hydrogen atom of the alcohol group in the following manner:
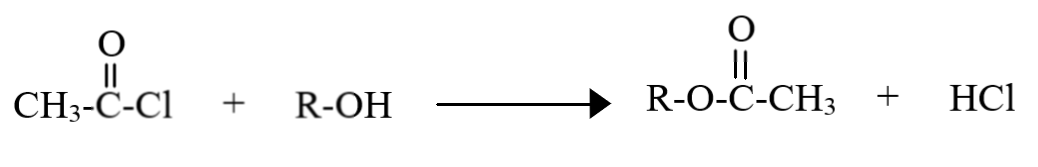
Thus, the group which gets attached is the acetyl group, having two carbon atoms (atomic mass $ = 12g/mol$), three hydrogen atoms (atomic mass $ = 1g/mol$) and one oxygen atom (atomic mass $ = 16g/mol$). Hence, the molecular mass of the acetyl group is:
$(12 \times 2) + (1 \times 3) + 16 = 43g/mol$
The mass of a hydrogen atom, as we know, is $1g/mol$. Therefore, the change in mass is caused by the leaving hydrogen atom and the incoming acetyl group. Thus, the increase in mass would be the difference between molecular masses of the acetyl group and hydrogen atom. Hence, the increase in mass caused by the addition of one acetyl group is:
$43 - 1 = 42g/mol$
Thus, dividing the total increase in mass with the increase in mass caused by the addition of a single acetyl group would give us the number of acetyl groups added, which is equal to the number of hydrogen atoms replaced, which is in turn equal to the number of ‘$OH$’ groups in the molecule. The total increase in mass is given as $126$ units. Therefore, dividing this with $42$, we get:
Number of ‘$OH$’ groups in the molecule $ = \dfrac{{126}}{{42}} = 3$
Hence, there are three ‘$OH$’ groups in the molecule. Therefore, the correct option is B.
Note:
Acetylation can be carried out using many reagents, like acetyl chloride (shown in the diagram), acetic anhydride etc. In the actual mechanism, note that the entire acetylating agent first gets added to the oxygen atom of the alcohol. Since this product is unstable, the group attached to the acetyl ion (chlorine in this case) abstracts the hydrogen, leaving behind a stable ester.
Complete step by step solution:
During acetylation of an alcohol, the acetyl group ($C{H_3}(CO)$) substitutes the hydrogen atom of the alcohol group in the following manner:

Thus, the group which gets attached is the acetyl group, having two carbon atoms (atomic mass $ = 12g/mol$), three hydrogen atoms (atomic mass $ = 1g/mol$) and one oxygen atom (atomic mass $ = 16g/mol$). Hence, the molecular mass of the acetyl group is:
$(12 \times 2) + (1 \times 3) + 16 = 43g/mol$
The mass of a hydrogen atom, as we know, is $1g/mol$. Therefore, the change in mass is caused by the leaving hydrogen atom and the incoming acetyl group. Thus, the increase in mass would be the difference between molecular masses of the acetyl group and hydrogen atom. Hence, the increase in mass caused by the addition of one acetyl group is:
$43 - 1 = 42g/mol$
Thus, dividing the total increase in mass with the increase in mass caused by the addition of a single acetyl group would give us the number of acetyl groups added, which is equal to the number of hydrogen atoms replaced, which is in turn equal to the number of ‘$OH$’ groups in the molecule. The total increase in mass is given as $126$ units. Therefore, dividing this with $42$, we get:
Number of ‘$OH$’ groups in the molecule $ = \dfrac{{126}}{{42}} = 3$
Hence, there are three ‘$OH$’ groups in the molecule. Therefore, the correct option is B.
Note:
Acetylation can be carried out using many reagents, like acetyl chloride (shown in the diagram), acetic anhydride etc. In the actual mechanism, note that the entire acetylating agent first gets added to the oxygen atom of the alcohol. Since this product is unstable, the group attached to the acetyl ion (chlorine in this case) abstracts the hydrogen, leaving behind a stable ester.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




