
Acetyl chloride cannot be obtained by treating acetic acid with:
A.\[{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\]
B.\[{\rm{PC}}{{\rm{l}}_{\rm{3}}}\]
C.\[{\rm{PC}}{{\rm{l}}_5}\]
D.\[{\rm{SOC}}{{\rm{l}}_{\rm{2}}}\]
Answer
232.8k+ views
Hint: Acetyl chloride is an acyl chloride originating from acetic acid.
It has no colour and is a volatile liquid.
Complete Step by Step Solution:
Acetyl chloride relates to the category of organic compounds called acid halides.
Acid halides are the compounds in which the -OH group of the carboxylic acid is replaced by a halide like chlorine, bromine, fluorine and iodine.
Acetyl chloride has the chemical formula\[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COCl}}\].
One of the methods of preparation of acetyl chloride is the treatment of acetic acid with compounds containing chlorine atoms.
A. \[{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\]
When chloroform is treated with acetic acid, acetyl chloride is not formed as a product.
So, A is correct.
B. \[{\rm{PC}}{{\rm{l}}_{\rm{3}}}\]
This is phosphorus trichloride.
When phosphorus trichloride is treated with acetic acid, acetyl chloride is formed as the product.
Phosphorous acid is formed as the side product.
The reaction occurs as follows:
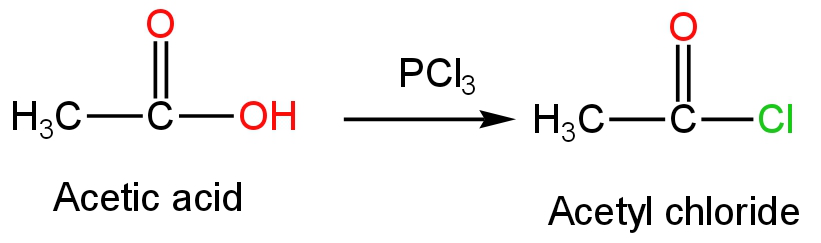
Image: Reaction of phosphorus trichloride with acetic acid.
So, B is incorrect.
C. \[{\rm{PC}}{{\rm{l}}_5}\]
This is phosphorus pentachloride.
When phosphorus pentachloride is treated with acetic acid, acetyl chloride is formed as the product.
Phosphorus oxychloride and hydrochloric acid are formed as side products.
The reaction occurs as follows:
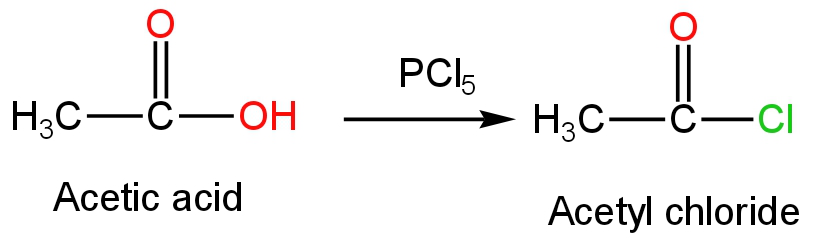
Image: the reaction of phosphorus pentachloride with acetic acid.
D.\[{\rm{SOC}}{{\rm{l}}_{\rm{2}}}\]
This is thionyl chloride.
When thionyl chloride is treated with acetic acid, acetyl chloride is formed as the product.
Sulfur dioxide and hydrochloric acid are formed as side products.
This reaction is preferred over other methods of conversion due to the by-products in these reactions being gases escaping leaving the acetyl chloride in an almost pure state.
The reaction occurs as follows:
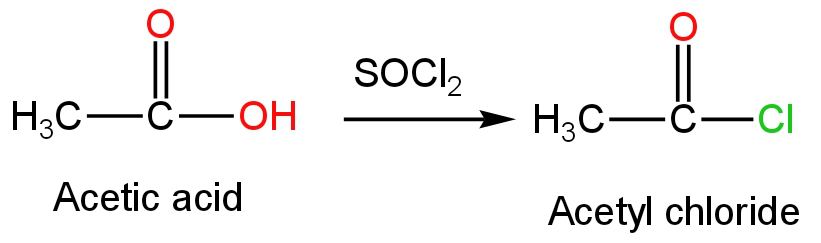
Image: the reaction of thionyl chloride with acetic acid.
So, option A is correct.
Note: While attempting the question, one must know about the structures of every given option. The oxidation of benzyl chloride out of the given options will yield benzoic acid. Oxidation of chlorophenol, chlorotoluene, and chlorobenzene will yield benzene diols, chlorobenzoic acid, and ortho-substituted oxy-chlorobenzene respectively.
It has no colour and is a volatile liquid.
Complete Step by Step Solution:
Acetyl chloride relates to the category of organic compounds called acid halides.
Acid halides are the compounds in which the -OH group of the carboxylic acid is replaced by a halide like chlorine, bromine, fluorine and iodine.
Acetyl chloride has the chemical formula\[{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{COCl}}\].
One of the methods of preparation of acetyl chloride is the treatment of acetic acid with compounds containing chlorine atoms.
A. \[{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\]
When chloroform is treated with acetic acid, acetyl chloride is not formed as a product.
So, A is correct.
B. \[{\rm{PC}}{{\rm{l}}_{\rm{3}}}\]
This is phosphorus trichloride.
When phosphorus trichloride is treated with acetic acid, acetyl chloride is formed as the product.
Phosphorous acid is formed as the side product.
The reaction occurs as follows:

Image: Reaction of phosphorus trichloride with acetic acid.
So, B is incorrect.
C. \[{\rm{PC}}{{\rm{l}}_5}\]
This is phosphorus pentachloride.
When phosphorus pentachloride is treated with acetic acid, acetyl chloride is formed as the product.
Phosphorus oxychloride and hydrochloric acid are formed as side products.
The reaction occurs as follows:

Image: the reaction of phosphorus pentachloride with acetic acid.
D.\[{\rm{SOC}}{{\rm{l}}_{\rm{2}}}\]
This is thionyl chloride.
When thionyl chloride is treated with acetic acid, acetyl chloride is formed as the product.
Sulfur dioxide and hydrochloric acid are formed as side products.
This reaction is preferred over other methods of conversion due to the by-products in these reactions being gases escaping leaving the acetyl chloride in an almost pure state.
The reaction occurs as follows:

Image: the reaction of thionyl chloride with acetic acid.
So, option A is correct.
Note: While attempting the question, one must know about the structures of every given option. The oxidation of benzyl chloride out of the given options will yield benzoic acid. Oxidation of chlorophenol, chlorotoluene, and chlorobenzene will yield benzene diols, chlorobenzoic acid, and ortho-substituted oxy-chlorobenzene respectively.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




